There continues to be ongoing discussion around the interpretation of the regulatory text – however for the purpose of this blog we are following the general consensus and the further detail as set out by MDCG 2020-5 Clinical Evaluation – Equivalence A guide for manufacturers and notified bodies
Annex XIV Part A (3) sets out the characteristics that need to be met:
The use of the word ‘similar’ again means that the characteristics in question are similar to such an extent that there is no clinically significant difference in the safety and performance of the device as a result of the differences.
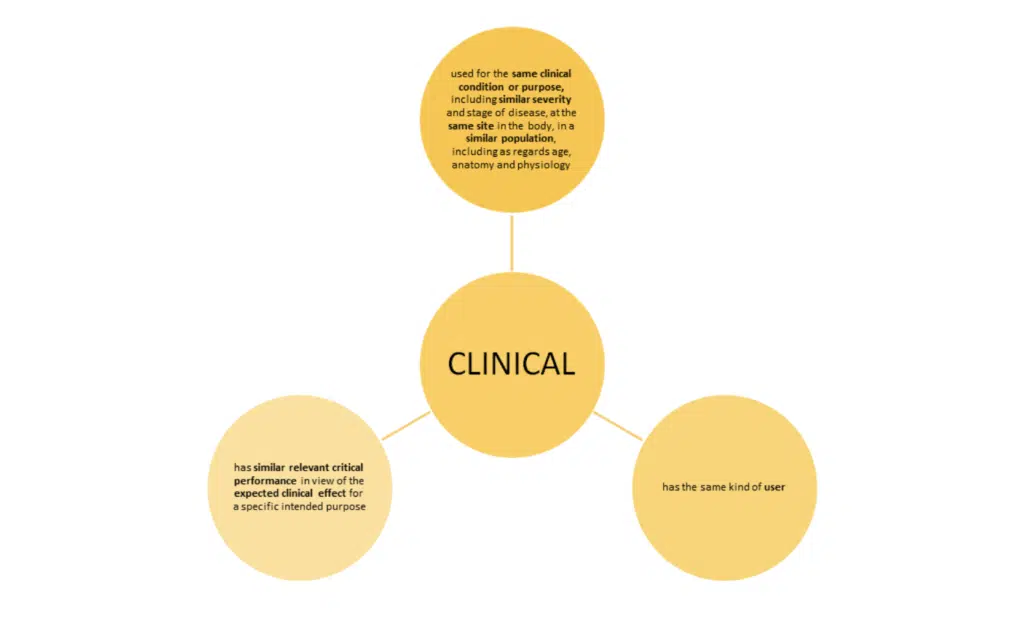
Note: ‘user’ means any healthcare professional or lay person who will use the device. It is important to consider the implication of the user’s level of competence on the safety and performance when considering equivalence.
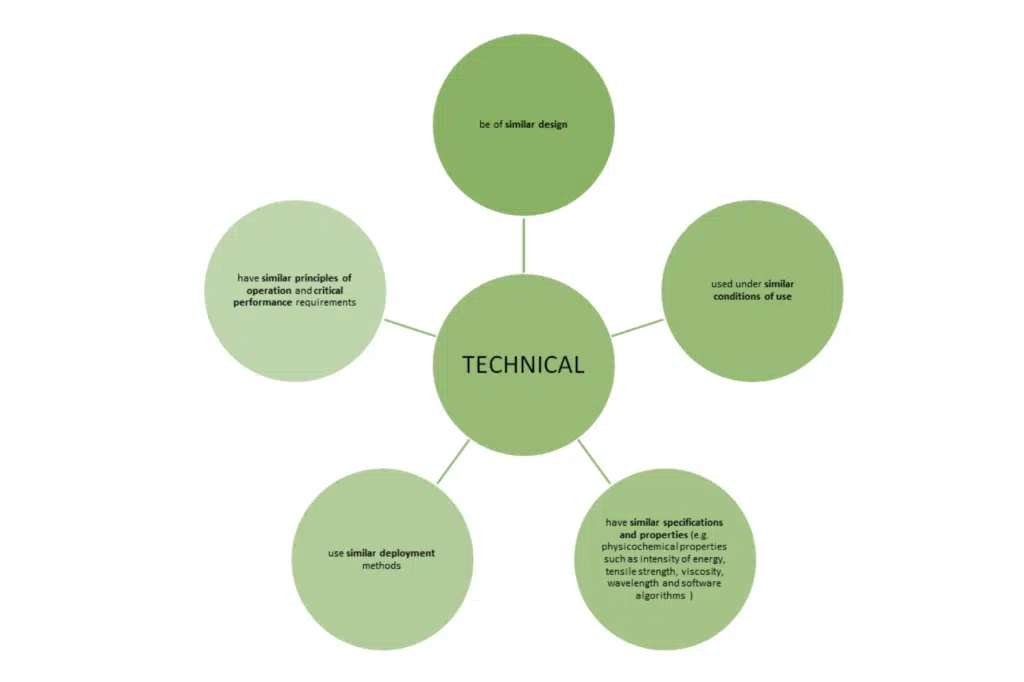
Note: the list of specifications and properties is not exhaustive, however important to note the clear inclusion of software algorithms in the MDR text. When reviewing the software algorithm, you should consider:
MDCG 2020-5 states that it is not reasonable to demand equivalence is demonstrated for the software code, as long as it have been developed in line with international standards for the design and validation of medical device software.
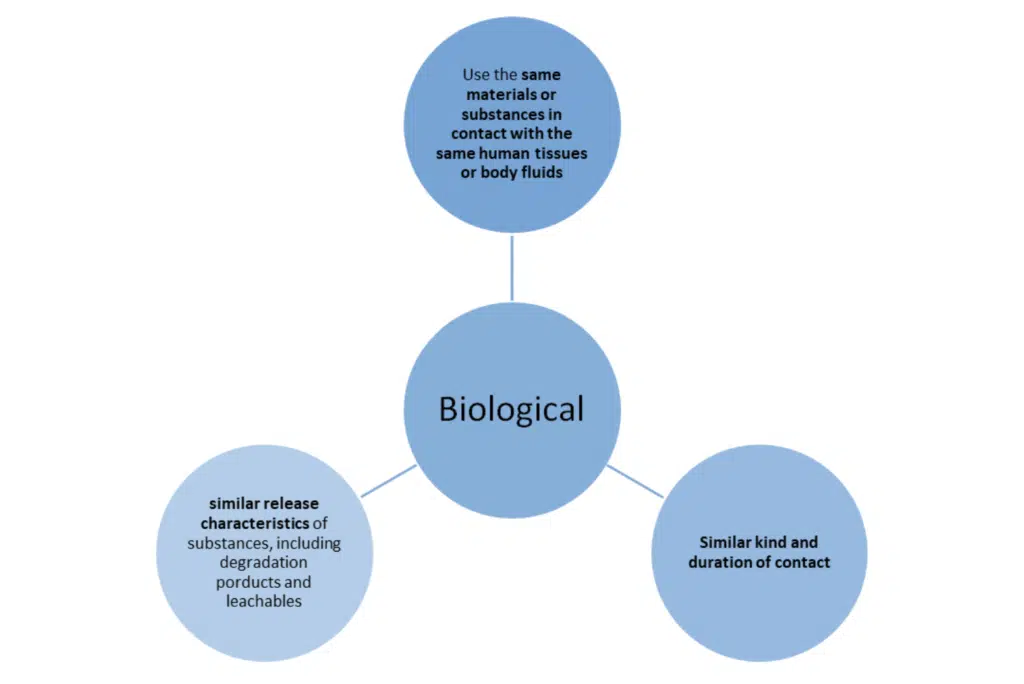
Note: in the exceptions previously set out in MEDDEV 27/1 revision 4 to not use the same material are not acceptable under the new regulations.
The ISO 10993 standards should be considered and can be adopted – some key sections to review:
Getting it right:
The MDR clarifies and adds in new requirements around the process of equivalence – below are some of the most important.
QMS – Annex IX, Chapter 1 2.2 (c) further tightens the regulations around equivalence, setting out the QMS requirements – manufacturers must set out their process for handling equivalence in their QMS.
Assessment & Gap analysis: Equivalence is part of the bigger clinical evaluation picture – manufacturers are required to specify and justify the level of clinical evidence that is necessary to demonstrate conformity with the general safety and performance requirements relevant to their device.
This must be based on robust scientific justification. With regard to equivalence this requires all three characteristics to be duly considered. All differences should be disclosed and evaluated.
Whilst some aspects of the characteristics must be the SAME, there are those that need only be SIMILAR, there must be a clear gap analysis performed and any differences evaluated.
Modifications: Modifications are common place however they do need to be considered carefully. MDCG sets out that if modifications are introduced to address a specific safety or performance concern, then the modifications are justified and that no additional clinically significant difference will occur.
More than one device? Unlike in MEDDEV 2.7/1 rev 4., the MDR allows for more than one equivalent device. If more than one device is to be used, then a full analysis of each product must be done and fully documented in the clinical evaluation report.
What should not be done is using different parts of different devices to pull together one equivalent device’. Although the MDCG guidance does set out that an exception could be made to this where a device has ‘stand-alone’ devices within it.
When seeking to claim equivalence for a medical device, a manufacturer cannot use a product without intended medical purpose (Annex XVI)
Implantables & Class III
Clinical investigations on the device under evaluation should be conducted for these risk groups – UNLESS the meet the conditions set out in Article 61 (4):
Equivalent device from another manufacturer? The conditions above need to be met but in addition the manufacturer must have a contract in place – allowing for full and ongoing access to the technical documentation of the equivalent device.
Devices other than implantables and class III: there is not stipulation that the equivalent device must have been assessed against the MDR – it is presumed devices marketed can be under the relevant Directive or the MDR. It may also be possible to claim equivalence to products without a CE mark – however the MDR requirements still must be met. The regulatory status of the product must be disclosed.
Whilst for these products a contract is not required, the manufacturer must still be able to demonstrate that they have sufficient access to the data that related to the equivalent device.
Devices composed of substances or of combinations of substances: where these substances are intended to be introduced into the human body, absorbed or locally dispersed in the human body – for the claim of equivalence, these substances are required to be the same.
Manufacturers should be aware that these products need to comply with Directive 2001/83/EC Annex I – this sets out the relevant requirements for: Evaluation of absorption, distribution, metabolism, excretion, local tolerance, toxicity, interaction with other devices, medicinal products or other substances and the potential for adverse reactions. Important to note that these must be considered as part of any claim of equivalence.
Medical devices & ancillary medicinal substance: When demonstrating equivalence and reviewing the biological characteristics requirement around the same materials or substances, this applies to any medicinal substance and any excipients or coatings as well.
As these products will be Class III – note the additional requirements on claiming equivalence set out above.
If the product under evaluation contains an ancillary medicinal substance, equivalence cannot be claimed against a device without an ancillary medicinal substance.
Products without an intended medical purpose: equivalence can be claimed against an analogous medical device – if there is clear justification. Otherwise these products are expected to have clinical investigations performed to generate the data. The three characteristics of equivalence should be followed. ‘Clinical benefit’ is interpreted as the need to demonstrate the performance.
Don’t forget to check any relevant common specifications for any additional requirements for that product group!
Documentation:
As set out throughout this blog – documentation is critical. MDCG 2020-5 provides manufacturers with table to utilise for the demonstration of equivalence, we’d encourage you to look and familiarise yourself with the table.
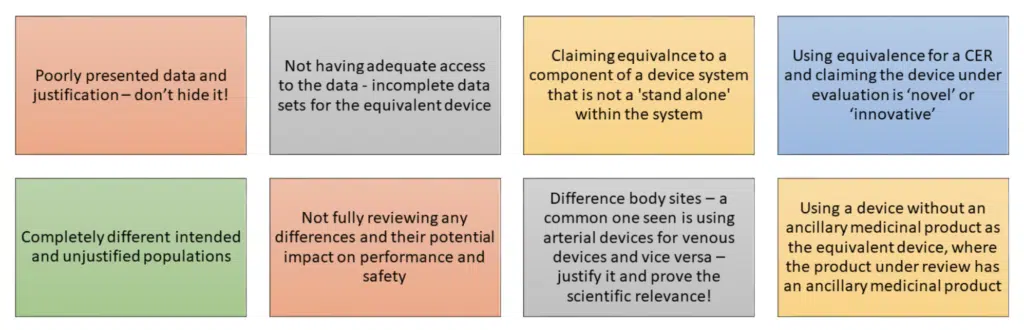
TAKE HOME MESSAGE:
We hope this has helped set out the requirements and considerations when looking to use equivalence in your CER. For the detailed text, refer to the MDR & MDCG 2020-5 and do get in touch if you have any further questions.
Subscribe to our newsletter

VISIT US
ECLEVAR FRANCE:
231 rue Saint-Honoré, 75001 Paris, France
ECLEVAR GMBH
ERFURT, Erfurt Hauptbahnhof
4th, 5th floor
Bahnhofstr. 38 Erfurt 99084
ECLEVAR Australia
Umina Beach NSW 2257, Australia
ECLEVAR UK Limited
3rd Floor 207 Regent Street, London, W1B 3HH
CONTACT US