The current UK MDR 2002 is based on the three EU directives, this is applicable to England, Scotland & Wales. As with the EU Directives, the use of equivalence continues to be a valid method for data generation for a clinical evaluation. Whilst not part of the current legal framework, the UK regulator, MHRA, and the UK Approved Bodies consider compliance with the principles of MEDDEV 2.7/1 revision 4 to be critical.
As such, there are similarities in the expectations when compared to the EU MDR (stay tuned for the next in this series).
MEDDEV 2.7/1 revision 4 provides guidance for manufacturers and notified/approved bodies on clinical evaluation – this blog is focused on Appendix I and the use of equivalence.
When used in line with Appendix I of MEDDEV 2.7/1 rev.4, data from equivalent devices can be a scientifically valid approach to clinical evaluation. However, it is sometimes seen as a ‘shortcut’ in the process. We hope this blog will help you understand the steps and some of the errors made.
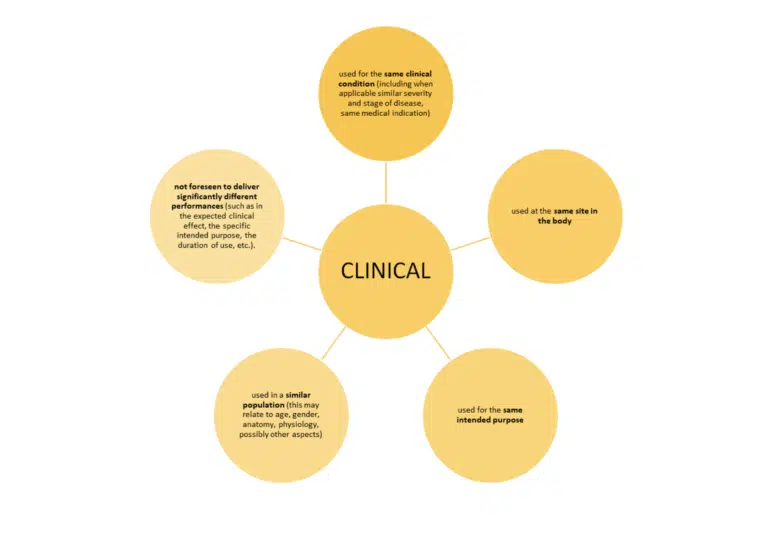
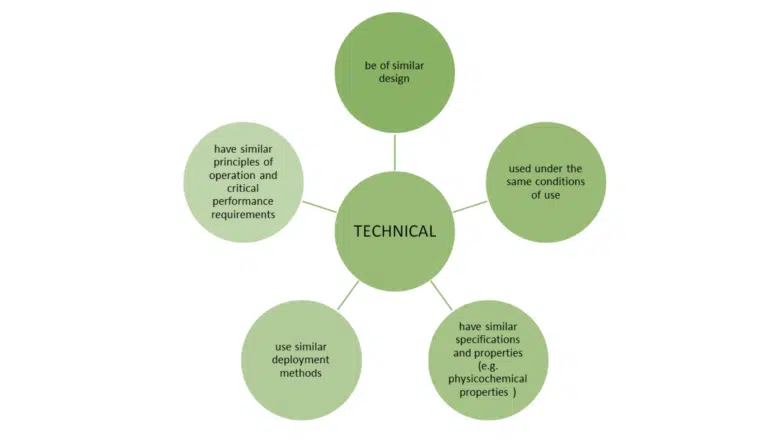
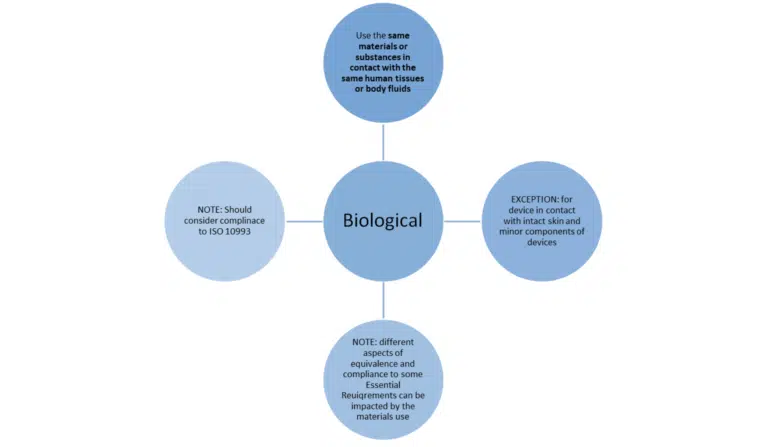
Just meeting these bullet points is not the whole story. MEDDEV 2.7/1 revision 4 sets out additional conditions when claiming equivalence to another device.
Attention to detail:
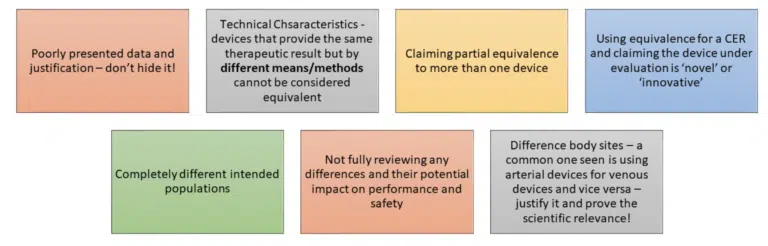
It is a scientific evaluation of data; it should be done thoroughly and used appropriately.
TAKE HOME MESSAGE:
If in doubt, read MEDDEV 2.7/1 revision 4 and ask an expert!
We hope this has helped set out the requirements and considerations when looking to use equivalence in your CER. For the detailed text, refer to MEDDEV 2.7/1 Revision 4 published in 2016.
Subscribe to our newsletter

VISIT US
ECLEVAR FRANCE:
231 rue Saint-Honoré, 75001 Paris, France
ECLEVAR GMBH
ERFURT, Erfurt Hauptbahnhof
4th, 5th floor
Bahnhofstr. 38 Erfurt 99084
ECLEVAR Australia
Umina Beach NSW 2257, Australia
ECLEVAR UK Limited
3rd Floor 207 Regent Street, London, W1B 3HH
CONTACT US