MDCG Guidance documents summary series:
MDCG 2029-9 Rev.1 – SSCP Summary of safety and clinical performance A guide for manufacturers and notified bodies
What is SSCP, who is involved, and what is the intention?
With the MDR 2017/745 Art. 32 manufacturers are required to provide a summary of safety and clinical performance (SSCP) for implantable devices and for class III devices, other than custom-made or investigational devices.
It is summary of clinical data and other information about the safety and clinical performance of the medical device
The SSCP will be an additional document to the instructions for use (IFU), implant cards or in any other mandatory documents. The SSCP shall be objective and summarize favorable and unfavorable data.
The SSCP will be addressed to professional users, but also to patients, where required (e.g. if an implant pass is required, if the patient is the user of the device) in the Member States national languages where the device will be on the market, and with appropriate wording so lay-persons can understand.
The notified body (NB) is requested to validate the SSCP and upload it onto EUDAMED, which means that this will be available publicly and free of charge in PDF format.
This is one step introduced by the MDR 2017/745 to enhance transparency and public information.
The MDCG set-up this guidance to assist and clarify the minimum requirements regarding presentation, content and validation of the SSCP.
The initial version was provided in September 2019, but was updated recently to provide some clarification on the Basic UDI-DI in association with the SSCP in EUDAMED (#3.1 pages 12, 13), and the manufacturer reference number to be covered in the template was added.
General requirements and recommendations for the SSCP
The Manufacturer is responsible for the accuracy of the SSCP.
Sources for the SSCP are:
- technical documentation (TD) like design verification/validation reports, the risk management report/file, the clinical evaluation report, and post-market surveillance (PMS) and post-market clinical follow-up (PMCF) plans and reports, PSUR.
- Since the IFU includes information from these sources also, it might be used as a source for the SSCP if appropriate.
A unique SSCP Reference number:
- Assigned by the manufacturer within the manufacturer’s management system
- will remain the same for the entire lifetime of the SSCP
- Serves as unique identification of the SSCP in EUDAMED and in EU when combined with the manufacturer’s SRN
- the SSCP shall be updated regularly and be up-to-date in EUDAMED.
The NB is the only party that can upload the SSCP onto EUDAMED.
If more than one NB is involved, the NB responsible for validation of the Technical Documentations is in charge of validation the SSCP.
What is also new is that the IFU shall contain all information needed to find the SSCP in EUDAMED. To be included in the IFU is the following (page 6):
- “It shall state that the SSCP is available in the European database on medical devices (Eudamed), where it is linked to the Basic UDI-DI.
- It should provide the URL to the Eudamed public website: https://ec.europa.eu/tools/eudamed
- It should state the value of the Basic UDI-DI. Alternatively, another metadata can be stated provided it can be used to unambiguously search and find the intended SSCP in Eudamed.”
There might be different language requirements for an IFU depending on whether the user is a health care professional or a patient, and Member States may have different requirements. This refers also to the SSCP.
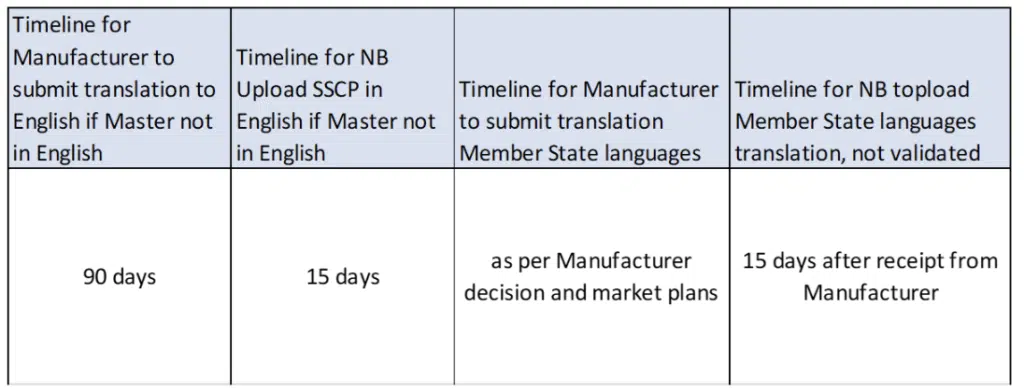
The SSCP can be in an EU Member State Language that the NB accepts (Master SSCP), but if this is another language than English, the Manufacturer needs to provide a translated English-Master version in addition to the NB. The Master SSCP will be validated by the NB, and then uploaded to EUDAMED.
The Manufacturer is responsible for the translation to English, and any translation to other EU languages where the device shall be marketed, including the verification of correctness of the translation. The NB will upload the translations onto EUDAMED, but not validate those.
For each language it should be a separate SSCP, including a statement about the language the SSCP was validated by the NB.
SSCP – Requirements, Validation, Upload, Translations and Responsibilities
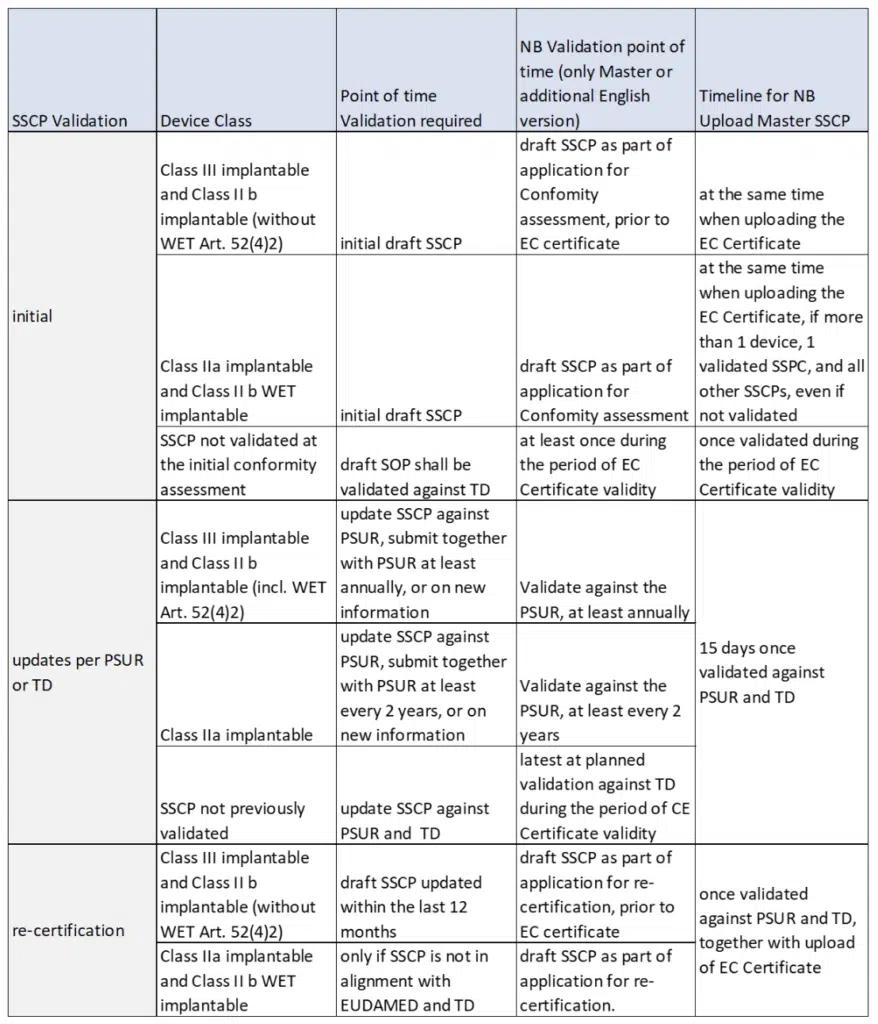
The SSCP draft will be submitted to the notified body (NB) for validation:
- At Initial Validation for conformity assessment, or for the first SSCP for old or legacy devices
- At re-certification of the conformity assessment, or no validated version of the SSCP is available
- When PMCF evaluation report and the periodic safety update report (PSUR) are updated
- When new information is available
Below is a summary of the Requirements and Tasks for Notified Bodies and Manufacturers:
Translation Management:
Guidance for each of the required sections of the SSCP document
The provisions for a SSCP template are given in the Appendix: Template for the SSCP, which includes the minimum required information.
The MDCG provide guidance for each required section. This is very informative and gives answers and clarification with regards to the expected information in the sections of the SSCP:
- The identification of the device and the manufacturer, including the Basic UDI-DI and, if already issued, the SRN
- The intended purpose of the device and any indications, contraindications and target populations
- A description of the device, including a reference to previous generation(s) or variants if such exist, and a description of the differences, as well as, where relevant, a description of any accessories, other devices and products, which are intended to be used in combination with the device
- Information on any residual risks and any undesirable effects, warnings and precautions
- The summary of clinical evaluation as referred to in Annex XIV, and relevant information on post-market clinical follow-up
- Possible diagnostic or therapeutic alternatives
- Suggested profile and training for users
- Reference to any harmonised standards and Common Specifications (CS) applied
- Revision history
The MDCG recommends to separate the information for intended users/healthcare professionals and for patients in two parts of the SSCP.
Other helpful MDCG Guidance
- MDCG2019-3 Rev. 1 Clinical evaluation consultation procedure exemptions Interpretation of article 54(2)b
- MDCG 2019-8 v2 Guidance document implant card on the application of Article 18 Regulation (EU) 2017/745 on medical devices
- MDCG 2019-11 Qualification and classification of software – Regulation (EU) 2017/745 and Regulation (EU) 2017/746
- MDCG 2019-16 rev.1 Guidance on cybersecurity for medical devices
- MDCG 2020-1 Guidance on clinical evaluation (MDR) / Performance evaluation (IVDR) of medical device software
- MDCG 2020-6 Guidance on sufficient clinical evidence for legacy devices
- MDCG 2020-7 Guidance on PMCF plan template
- MDCG 2020-8 Guidance on PMCF evaluation report template
- MDCG 2020-10-1 Guidance on safety reporting in clinical investigations
- MDCG 2020-10-2 Clinical Investigation Summary Safety Report Form v1.0
- MDCG 2020-13 Clinical evaluation assessment report template (and MEDDEV 2.7/1 rev. 4)
- The MDCG 2021-1 Rev.1 Guidance on harmonised administrative practices and alternative technical solutions until EUDAMED is fully functional (respectively referrals to: Art. 70, Art. 73, Art. 74, Art. 75, Art. 76, Art. 77, Art. 78, Art. 80), see also our blog: Summary of the MDCG Guidance Document MDCG 2021-20
- MDCG 2021-6 Regulation (EU) 2017/745 – Questions & Answers regarding clinical investigation
- MDCG 2021-8 Clinical investigation application/notification documents, see also our blog: Summary of the MDCG Guidance Document MDCG 2021-8.
- MDCG 2021-11 Guidance on Implant Card – Device types
- MDCG 2021-20 Instructions for generating CIV-ID for MDR Clinical Investigations, see also our blog: Summary of the MDCG Guidance Document MDCG 2021-20
- MDCG 2021-25 Application of MDR requirements to “legacy devices” and to devices placed on the market prior to 26 May 2021 in accordance with Directives 90/385/EEC or 93/42/EEC
- MDCG 2021-28 Substantial modification of clinical investigation under Medical Device Regulation, see also our blog: Summary of the MDCG Guidance Document MDCG 2021-28
- MDCG Guidance Documents in the section Notified Bodies
- MDCG Guidance Documents in the section Unique Device Identifier (UDI)
Helpful guidance is provided for the Classification of Devices and Combination Products Medical Device and Medicinal Product:
- MDCG 2022-5 Guidance on the borderline between medical devices and medicinal products under Regulation (EU) 2017/745 on medical devices
- MDCG 2021-24 Guidance on classification of medical devices
- Exchange of information between medical device competent authorities on borderline and classification cases Helsinki Procedure 2021
In addition, helpful guidance is provided for “actors” registration under MDR:
- MDCG 2019-4 Timelines for registration of device data elements in EUDAMED
- MDCG 2019-5 Registration of legacy devices in EUDAMED
- MDCG 2020-15: MDCG Position Paper on the use of the EUDAMED actor registration module and of the Single Registration Number (SRN) in the Member States
- MDCG 2021-13 rev.1: Questions and answers on obligations and related rules for the registration in EUDAMED of actors other than manufacturers, authorised representatives, and importers subject to the obligations of Article 31 MDR and Article 28 IVDR
- MDCG 2022-12 Guidance on harmonised administrative practices and alternative technical solutions until Eudamed is fully functional (for Regulation (EU) 2017/746 on in vitro diagnostic medical devices)
Guidance on Device Nomenclature
- MDCG 2018-2 Future EU medical device nomenclature – Description of requirements
- MDCG 2021-12 FAQ on the European Medical Device Nomenclature (EMDN)
- The EMDN – The nomenclature of use in EUDAMED
- The CND nomenclature – Background and general principles
Thank you for your time. If you found this interesting, please check-out for out next blog on the MDCG Document summaries: MDCG 2022-5 Guidance on borderline between medical devices and medicinal products under Regulation (EU) 2017/745 on medical devices

