A 510(k) is the technical dossier required by the US Food and Drug Administration (FDA) to sell a medium-risk medical device or IVD in the United States.
If you wish to place a device on the US market and a Premarket Approval Application is not required – you must submit a 510(k) to the FDA *note there are exemptions & limitations to this ‘.9 of the device classification regulation chapters (21 CFR 862.9, 21 CFR 864.9)’
The main principle of this submission is to demonstrate that your product is as ‘safe and effective’ (substantially equivalent) to a device that is legally marketed in the US. The legally marketed device or devices are referred to as the predicate devices(s).
You cannot market your device until the FDA have deemed it to be substantially equivalent – this decision is usually made within 90 days and is solely based on the information you provide in your submission.
510k submission cleared by the FDA are published – usually in the first week of the following month.
What counts as a predicate device?
It is possible to use more than one predicate, times this is utilized include:
If there is no appropriate predicate device, then manufacturers will need to submit a De Novo request or for high risk products a Premarket Approval may be required.
The approach to demonstrating equivalence is different to the EU/UK and has two key considerations: intended use and technological characteristics.
Below shows the two different ways in demonstrating this.
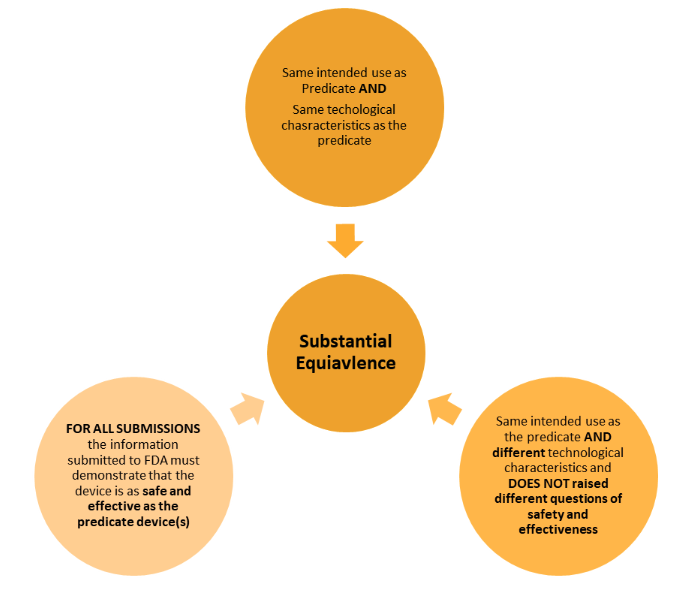
There are less strict requirements within the regulations when compared to the EU approach – however the principles are very much the same. The similarities and the differences must be clearly set out and evaluated.
There must be clear evidence to demonstrate the use of the predicate device(s) is valid and that there is assurance that the safety and effectiveness are substantially equivalent.
The onus is on the manufacturer to ensure this is presented in a robust and scientifically valid way.
This installment will turn the attention to the US and the FDA’s use of equivalence in us
Common reasons for Not Substantially Equivalent (NSE) decision
If an NSE decision is given – the manufacturer is given the chance to respond to the FDA determination & the deficiencies noted.
Take home message:
This is a useful route for the US market that offers more timely access to market
It should not be seen as a short cut – assurance around safety and performance is critical.
Provide clear and robust evidence in the submission. Only use legally marketed devices as a predicate
We hope this has helped set out the requirements and considerations when looking to use the FDA’s 510(k) route.
For further guidance on a evaluating substantial equivalence for your device you can refer to the FDA’s guidance

Subscribe to our newsletter

VISIT US
ECLEVAR FRANCE:
231 rue Saint-Honoré, 75001 Paris, France
ECLEVAR GMBH
ERFURT, Erfurt Hauptbahnhof
4th, 5th floor
Bahnhofstr. 38 Erfurt 99084
ECLEVAR Australia
Umina Beach NSW 2257, Australia
ECLEVAR UK Limited
3rd Floor 207 Regent Street, London, W1B 3HH
CONTACT US