Clinical Investigation with Medical Devices in Europe
Pre-CE mark Explanatory or Confirmatory Clinical Investigation,
or Post-market Clinical Investigations
Introduction
Recently ECLEVAR posted a blog about the response to TGA Consultation of 17th of August 2022 on proposed regulatory changes for clinical trials of medical devices [Regulatory changes for clinical trials of medical devices (eclevarmedtech.com)], highlighting the need to clarify TGA’s understanding of “novel” medical devices, and linking to ISO 14155:2020 and FDA Guidance for Industry: Investigational Device Exemptions (IDEs) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies of October 1st, 2013.
The definitions provided by ISO 14155:2020 in Annex I and the FDA Guidance, are almost congruent, whereas the FDA Guidance provides further guidance on the differences between early feasibility and traditional feasibility studies.
While the FDA guidance relates specifically to early feasibility study IDEs only, covered by the early feasibility study (EFS) program, there is currently no pendant in the EU. If there is a series of early feasibility clinical investigation (CI) for a medical device in the EU, the clinical investigation needs to be submitted MDR 2017/745 Art. 62 requirements as an own-standing CI, but references to previous CIs, including a summary of changes to the device design, are taken into consideration by the competent Authorities.
This whitepaper provides an overview on the different types of clinical investigations in the EU per MDR 2017/745, the submission routes and submission documents, and also on reporting requirements during conduct, and after completion of a clinical investigation. This whitepaper is based on current practice, which means in the absence of EUDAMED. Until EUDAMED is functionally, coordinated single submissions, SAE reporting and notifications via EUDAMED will not be possible.
Considerations when Planning a Clinical Investigation
PRE-MARKET CLINICAL INVESTIGATION AS PER ISO 14155:2020
With the updated ISO 14155:2020, the ISO incorporates a new Annex I, on the clinical development stages. Next to the regulatory status of the device at the timepoint of the clinical investigation, pre-market and post-market status, ISO 14155:2020 groups the CIs also depending on the clinical development stage, the type of design, the descriptors of the clinical investigation, and the burden to subjects.
Under the pre-market clinical investigations, ISO 14155:2020 Annex I divide clinical investigations into pilot stage and pivotal stage.
Regarding the burden on subjects, any pre-market clinical investigation is considered to be interventional.
CLINICAL DEVELOPMENT STAGE AND TYPE OF DESIGN
In the pre-market status, the clinical investigations are either the pilot stage or the pivotal stage. The type of design may be explanatory or confirmatory.
Identifiers for exploratory clinical investigations are, that they “might not have pre-specified primary hypotheses, and can be conducted to generate hypotheses, to be confirmed in subsequent clinical investigations.”
A confirmatory clinical investigation is “an adequately controlled clinical investigation in which the hypotheses of the primary endpoint(s) are stated before the start of the clinical investigation in the CIP and are analysed by the CIP (i.e. sound confirmative statistical testing is pre-specified, intended, and applied).”
The pilot stage clinical investigations can be exploratory or confirmatory.
These can be:
- I.5.2 First in human clinical investigations: “A clinical investigation in which a medical device for a specific indication is evaluated for the first time in human subjects.”
- I.5.3 Early feasibility investigations: “A limited clinical investigation of a device early in development, typically before the device design has been finalized, for a specific indication (e.g. innovative device for a new or established intended use, marketed device for a novel clinical application). It can be used to evaluate the device design concept concerning initial clinical safety and device clinical performance or effectiveness (if appropriate) as per intended use in a small number of subjects when this information cannot practically be provided through additional nonclinical assessments or appropriate nonclinical tests are unavailable. Information obtained from an early feasibility clinical investigation can guide device modifications. An early feasibility clinical investigation does not necessarily involve the first clinical use of a device.
NOTE Early feasibility clinical investigation can also be called proof of concept clinical investigation.”
- I.5.4 “Traditional feasibility investigations: A clinical investigation that is commonly used to capture preliminary clinical performance, effectiveness, or safety information of a near-final or final device design to adequately plan an appropriate pivotal clinical investigation. Because the clinical investigation of a near-final or final device design takes place later in development than an early feasibility clinical investigation, more non-clinical or prior clinical data are expected than in an early feasibility clinical investigation. A traditional feasibility clinical investigation does not necessarily need to be preceded by an early feasibility clinical investigation.”
The pivotal stage clinical investigations are only confirmatory clinical investigations:
- I.5.5 “Pivotal clinical investigation: A confirmatory clinical investigation designed to collect data on the clinical performance, effectiveness, or safety of a device for a specified intended use, typically in a statistically justified number of human subjects. It can or cannot be preceded by an early and/or a traditional feasibility clinical investigation.”
POST-MARKET CLINICAL INVESTIGATIONS AS PER ISO 14155:2020
As per ISO 14155:2020 Annex I, section I.2.3, a post-market clinical investigation is:
- “A clinical investigation carried out following market approval of a medical device, intended to answer specific questions relating to clinical performance, effectiveness or safety of a medical device when used in accordance with its approved labelling.”
- “If marketed medical devices are being investigated for new indications, other than described in the approved labelling, requirements for pre-market clinical investigations apply.”
Clinical investigations at post-market stage can be confirmatory and interventional, or observational and non-interventional observational. Observational clinical investigations may collect prospective or retrospective data. An example for the confirmatory, interventional post-market clinical investigation is the PMCF clinical investigation as per Art. 74 (1) MDR 2017/745.
With post-market clinical investigations, data of a broader subject population, the real-world use, long-term outcomes, ore rare adverse events can be investigated.
What needs to be considered for non-interventional clinical investigations, like registries, observational studies, or survey studies, is the non-interventional assignment process to a device.
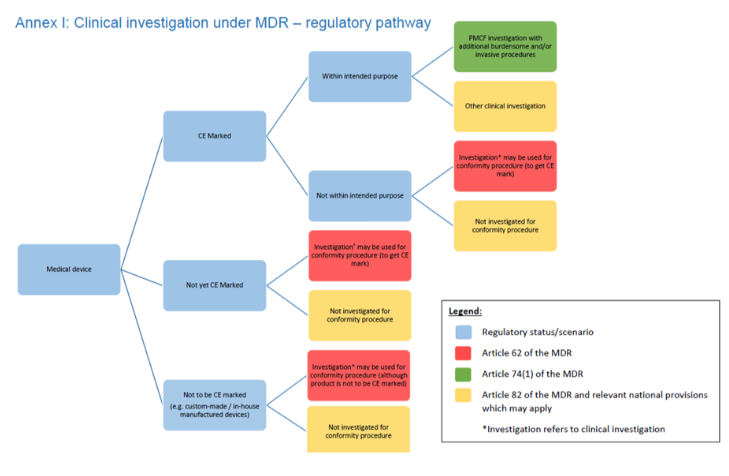
To close the loop of the process it is needed to refer to MDR 2017/745 for the selection of the correct CI submission route. The principle of ISO 14155:2020 Annex I would apply all clinical investigations, although the invasiveness would not. Investigations as per MDR 2017/745 Art. 62 and Art. 74 (2), some of the Art. 82 other clinical investigations, but also Article 74 (1) PMCF Clinical Investigations are considered invasive clinical investigations:
- Article 62: General requirements regarding clinical investigations conducted to demonstrate the conformity of devices– considered pre-market stage
- Article 74 (2): Clinical investigations regarding devices bearing the CE marking - outside the scope of its intended purpose – considered pre-market stage
- Article 74 (1) PMCF clinical investigations fall under the post-market stage, although the confirmatory design and interventional aspects need to be taken into account for planning these studies also
- Article 82: Requirements regarding other clinical investigations, if:
- It is an interventional clinical investigation with a CE-marked or non-CE marked device, but not for purposes of Art. 62.1 (example: CE marked Stent, investigation to explore the impact on blood flow; non-CE marked smart monitoring device, investigation to explore changes in the behavior of subjects when using the device)
On the figure of MDCG 2021-6 Annex I the decision pathway is made very visual:
While Art. 82 clinical investigations with CE-marked devices within the scope of intended use and without any additional burden to the subject, fall under the post-market stage (observational)