Equivalence in UK – Great Britain – How to use it!
Different regulatory jurisdictions utilise ‘equivalence’ in different ways, from forming part of a clinical evaluation to offering a direct route to market. We are going to consider its use across the globe, starting with Great Britain.
The current UK MDR 2002 is based on the three EU directives, this is applicable to England, Scotland & Wales. As with the EU Directives, the use of equivalence continues to be a valid method for data generation for a clinical evaluation. Whilst not part of the current legal framework, the UK regulator, MHRA, and the UK Approved Bodies consider compliance with the principles of MEDDEV 2.7/1 revision 4 to be critical.
As such, there are similarities in the expectations when compared to the EU MDR (stay tuned for the next in this series).
MEDDEV 2.7/1 revision 4 provides guidance for manufacturers and notified/approved bodies on clinical evaluation – this blog is focused on Appendix I and the use of equivalence.
When used in line with Appendix I of MEDDEV 2.7/1 rev.4, data from equivalent devices can be a scientifically valid approach to clinical evaluation. However, it is sometimes seen as a ‘shortcut’ in the process. We hope this blog will help you understand the steps and some of the errors made.
HOW DO YOU DEMONSTRATE EQUIVALENCE IN UK? – The three characteristics
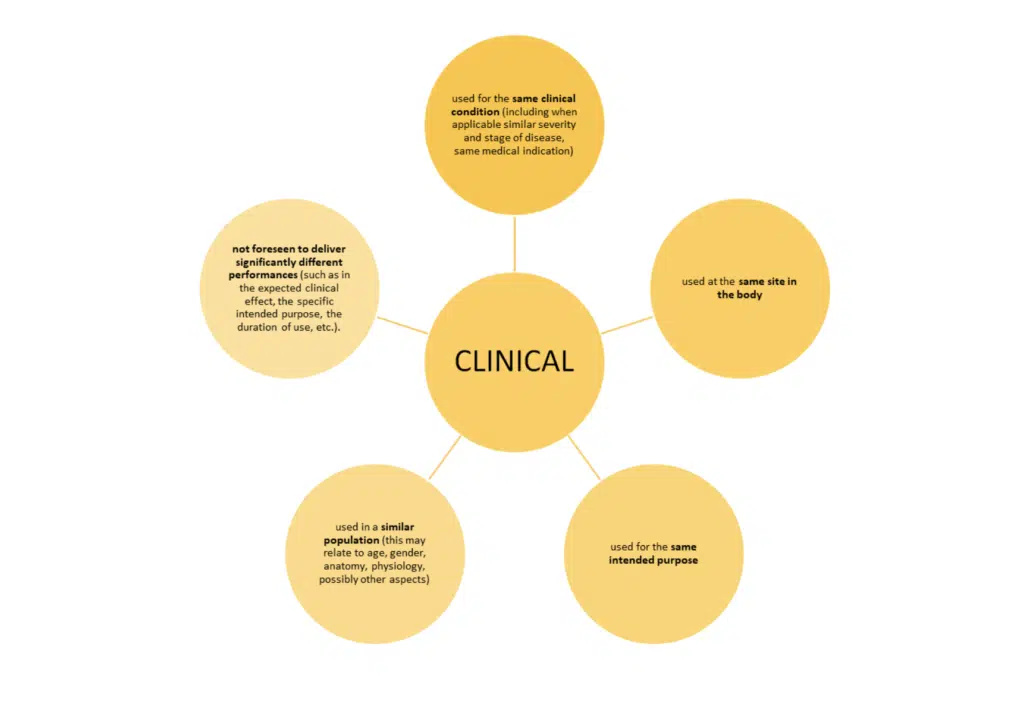
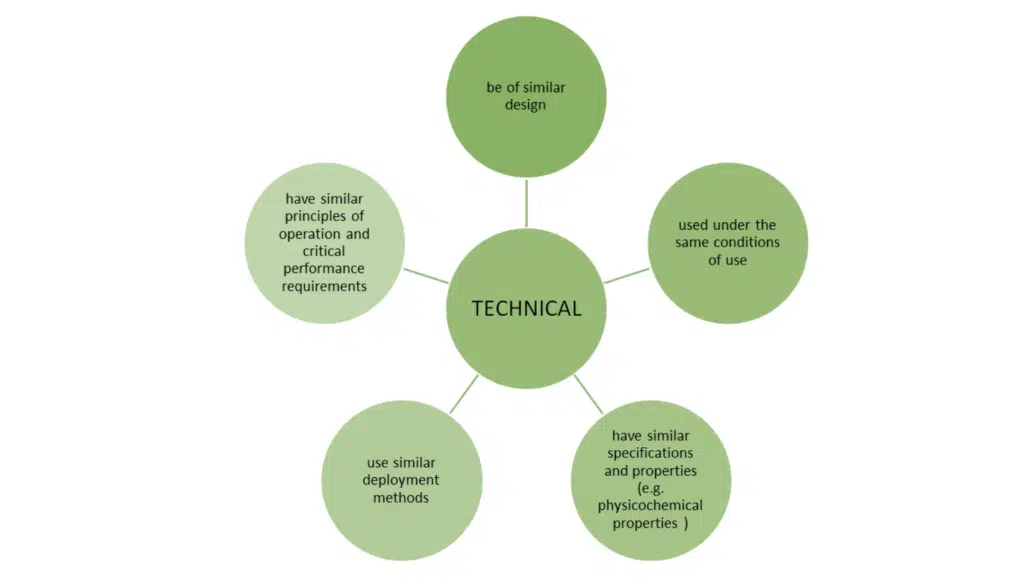
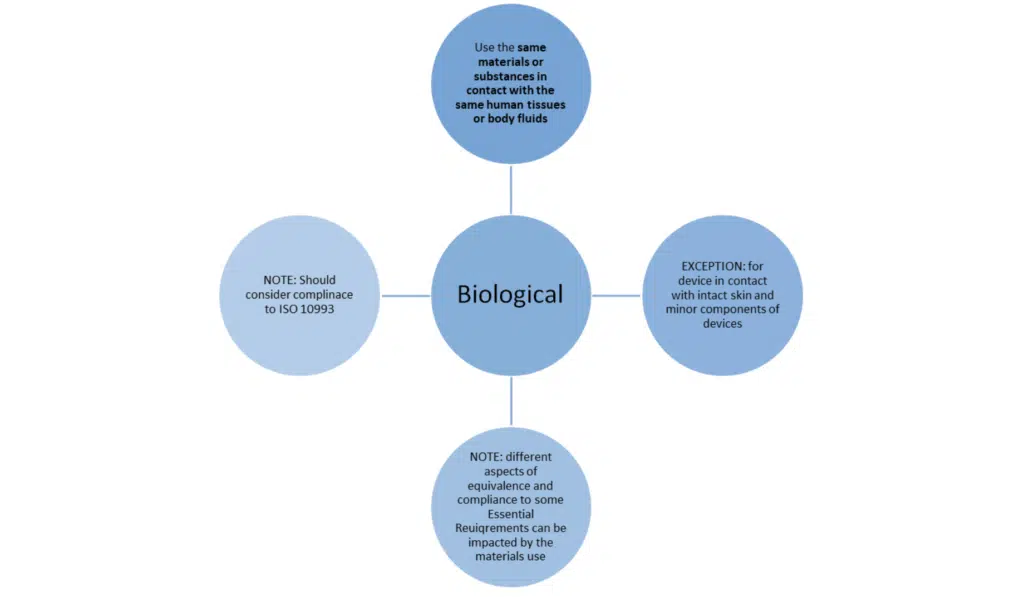
Just meeting these bullet points is not the whole story. MEDDEV 2.7/1 revision 4 sets out additional conditions when claiming equivalence to another device.
- Equivalence can only be claimed against a single device
- All three of the above characteristics must be fulfilled
- ‘SIMILAR’ – this means that any differences between the device under evaluation and the equivalent devices do not cause a clinically significant difference in the performance and safety of the device.
- The basis of the claim of equivalence must be identified and documented. A ‘gap analysis’ of any differences should clearly demonstrate those differences and explain why they are not clinically significant.
Equivalence in UK, attention to detail:
- Measurements: if possible, then clinically relevant specifications and properties should be measured for both the equivalent device and the device under evaluation – ideally in a comparative table.
- Manufacturing processes: how has the equivalent device been manufactured? Any special treatments such as surface modification. If so, what impact is there upon the technical and biological characteristics? This should be assessed and documented.
- Pictures: comparative pictures of the device under evaluation and the equivalent device should be provided in the CER.
- Non-clinical information: supporting data such as pre-clinical study reports, bench testing should be provided in the technical documentation and summarised in the CER.
- Biological characteristics: Often where data is missing.
- As mentioned above, the impact of manufacturing processes should be reviewed.
- There may be a need for histopathological studies demonstrating the same host response.
- If using data from animal studies, the choice of test and the predictive value need to document and justified.
- Consider if an ISO 10993-18 (Chemical characterisation) Annex C is needed.
- Abrasion and host response to particles should be considered.
WHAT CAN GO WRONG? This is a short list of common errors/challenges seen when using equivalence in uk.
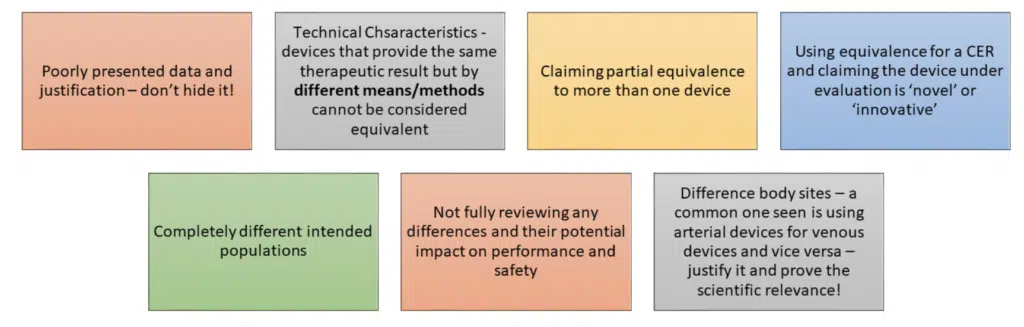
It is a scientific evaluation of data; it should be done thoroughly and used appropriately.
TAKE HOME MESSAGE:
- Fulfil the 3 criteria
- Document & demonstrated the evidence and justification
- Provide as much detail as possible.
- Carefully consider if this is the best route:
- does it provide enough evidence for the Clinical evaluation report?
- Has it demonstrated conformity for the device under evaluation?
- Does it address all the relevant Essential Requirements?
If in doubt, read MEDDEV 2.7/1 revision 4 and ask an expert!
We hope this has helped set out the requirements and considerations when looking to use equivalence in your CER. For the detailed text, refer to MEDDEV 2.7/1 Revision 4 published in 2016.
If you have any further questions do get in touch.

