
LSI Europe: A reflection on routes to market.
Eclevar Medtech was proud to sponsor LSI’s hugely successful event in London. It was an opportunity to meet and connect with a wide range of innovators, investors and key experts in the field.
We were able to host 2 panels, the first being on Route to Market for medical devices. An interesting session offering a high- level look at the key checkpoints on the journey to market access.
Some of the questions asked during and following the session were – where do I start with classification? What’s different under the EU MDR?
Determining the correct classification is KEY! It dictates your next steps and requirements in bringing a product to market.
Medical device classification is based on the devices intended use, characteristics and its inherent risks.
THE CATEGORIES:
Class I
- Class I
- Class Is
- Class Im – measuring function
- Class Ir – reusable Ir – reusable surgical instrument
Class IIa
Class IIb
- Class IIb
- Class IIb implantable
Class III
The classification rules sit in Annex VIII of Regulation 2017/745
Under the new regulations we see an increase in the rules compared to the MDD 9/42/EEC – there are 22 classification rules split in to 4 groups:
- Non-invasive medical devices (Rules 1-4)
- Invasive medical devices (Rules 5-8)
- Active medical devices (Rules 9-13)
- Special rules (Rules 14-22)
How to determine a classification
There is additional support in MDCG 2021-24 – Guidance on classification of medical devices
It is critical to have a clear understanding of the device and the following aspects:
- Specific medical purpose
- Duration of use: is it transient, short term or long term?
- Is the device invasive?
- Is it an active medical device?
- Does is have a measuring function?
- Do you have a system or procedure pack?
It is the intended use of a device that determines the classification – not any ‘off-label’ use that may occur.
The rules should be considered in order to establish the proper classification, it is very possible that more than one rule will apply. For example, an active medical device may also have general rules that also apply.
Where a device is covered by more than one rule it is the strictest rule or sub- rule that gives the highest classification that is applicable.
If you are unsure, seek support – for formal determination Competent Authorities can be approached. Note some charge for this service!
Here are the rules!
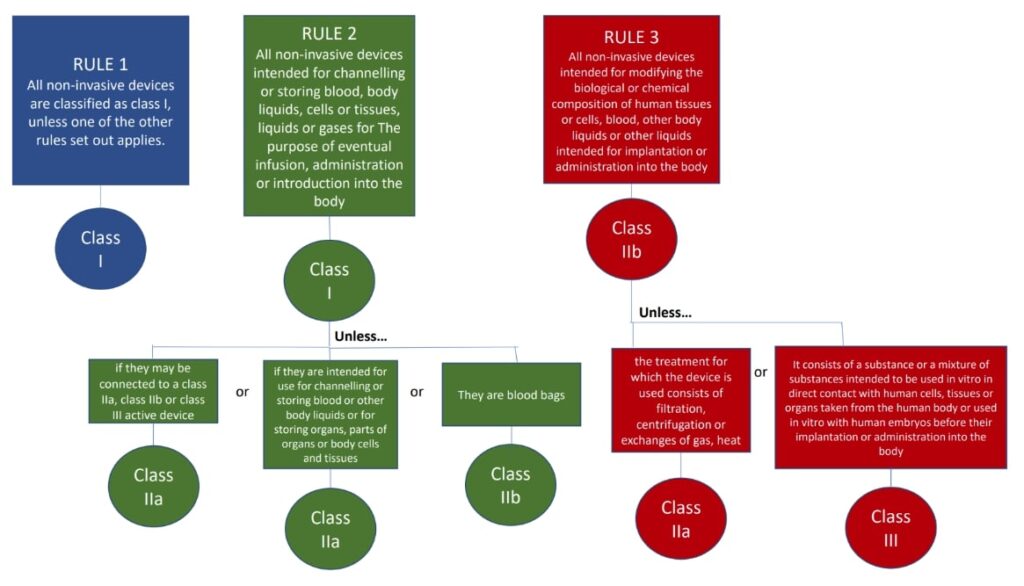
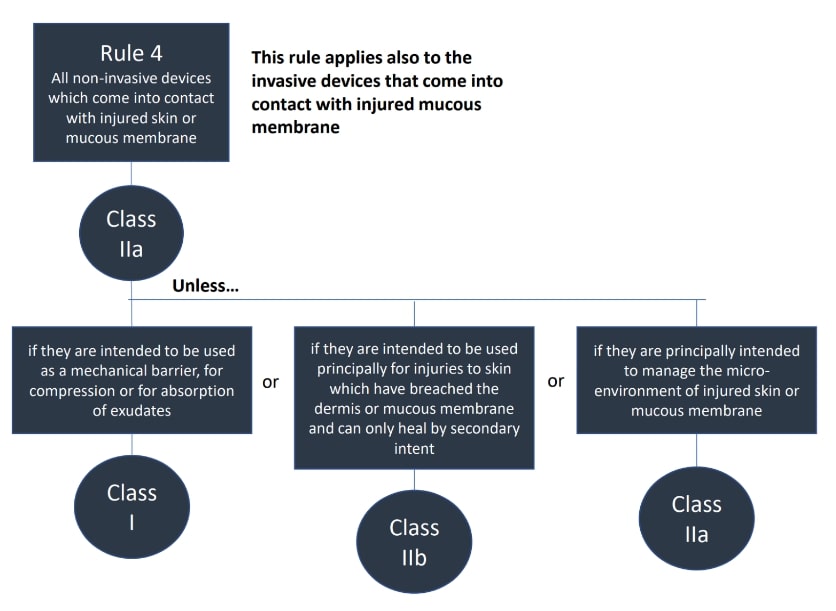
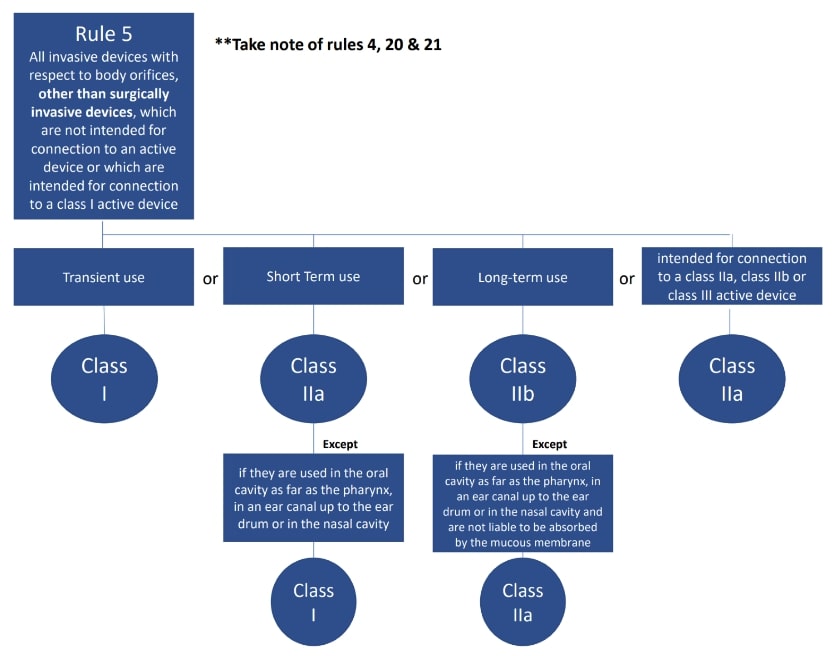
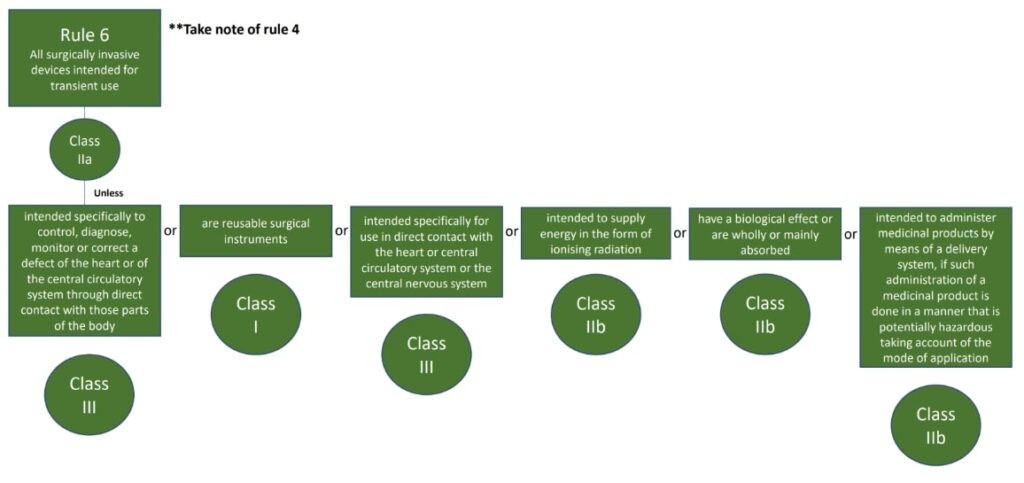
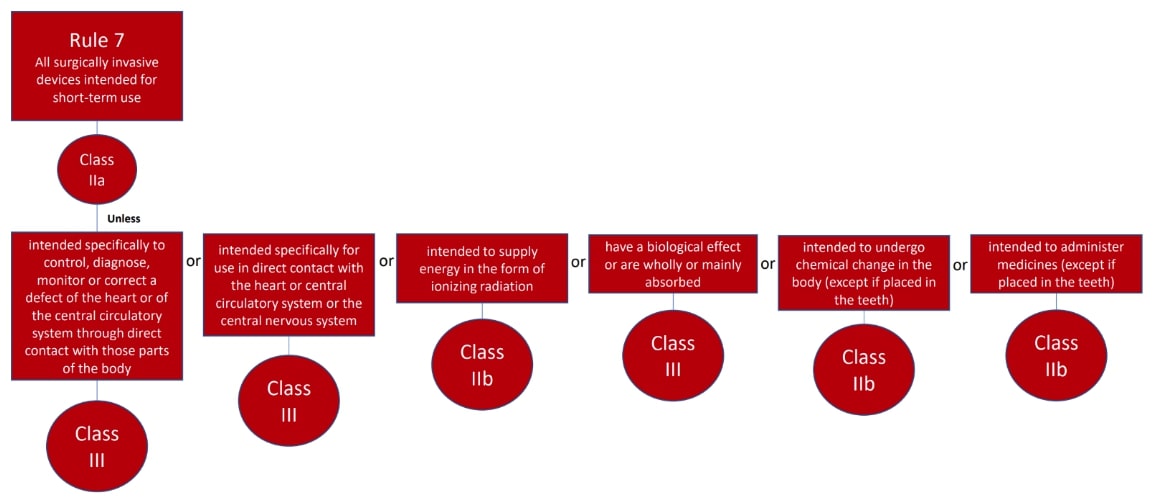
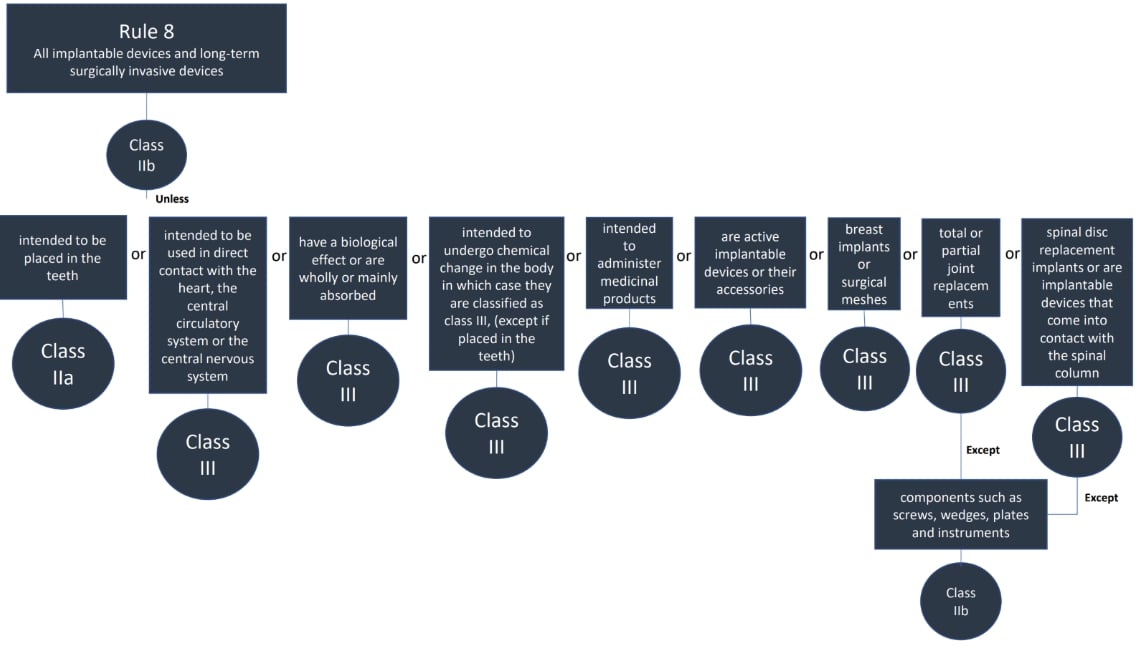
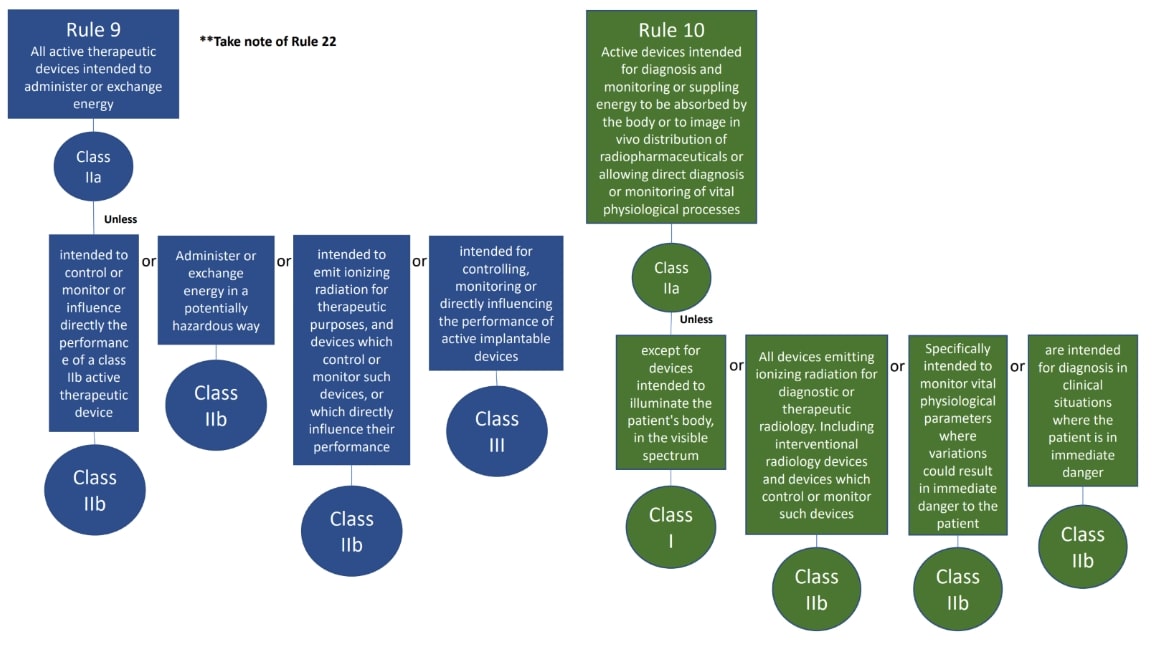
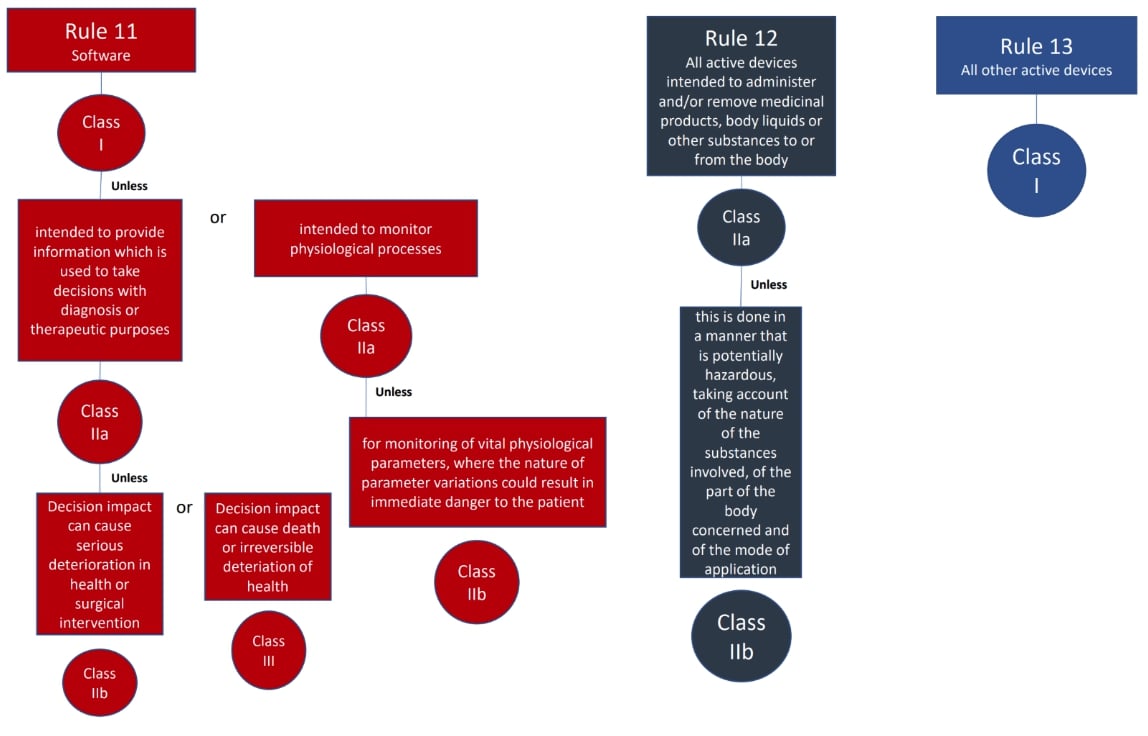
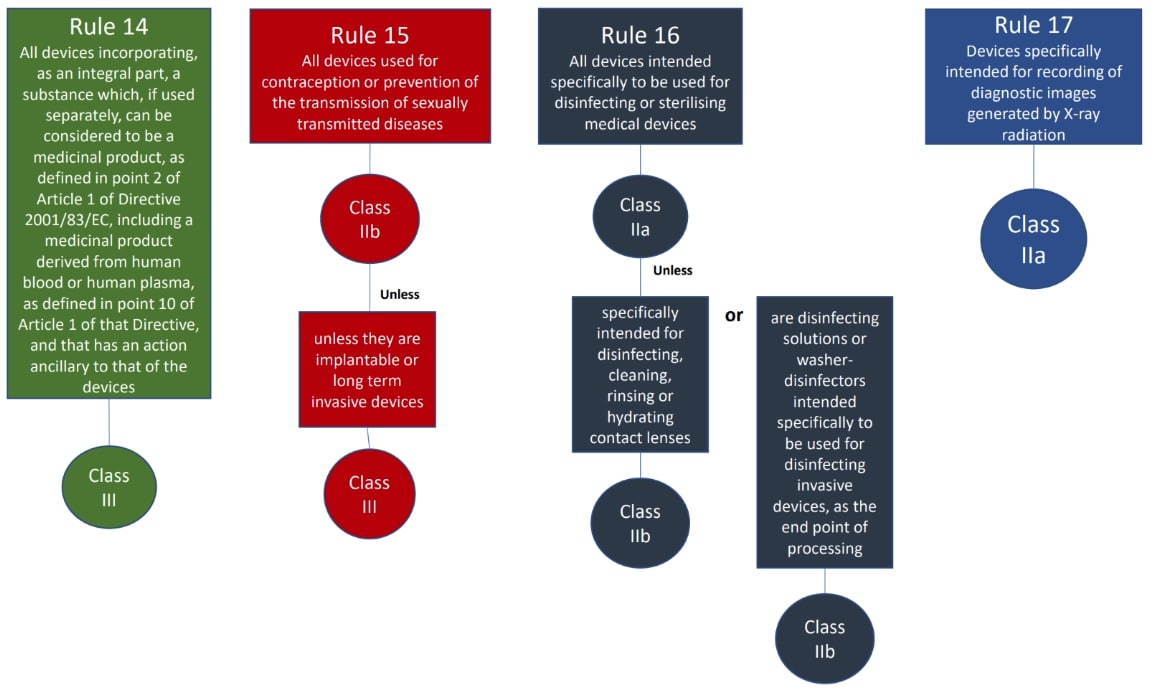
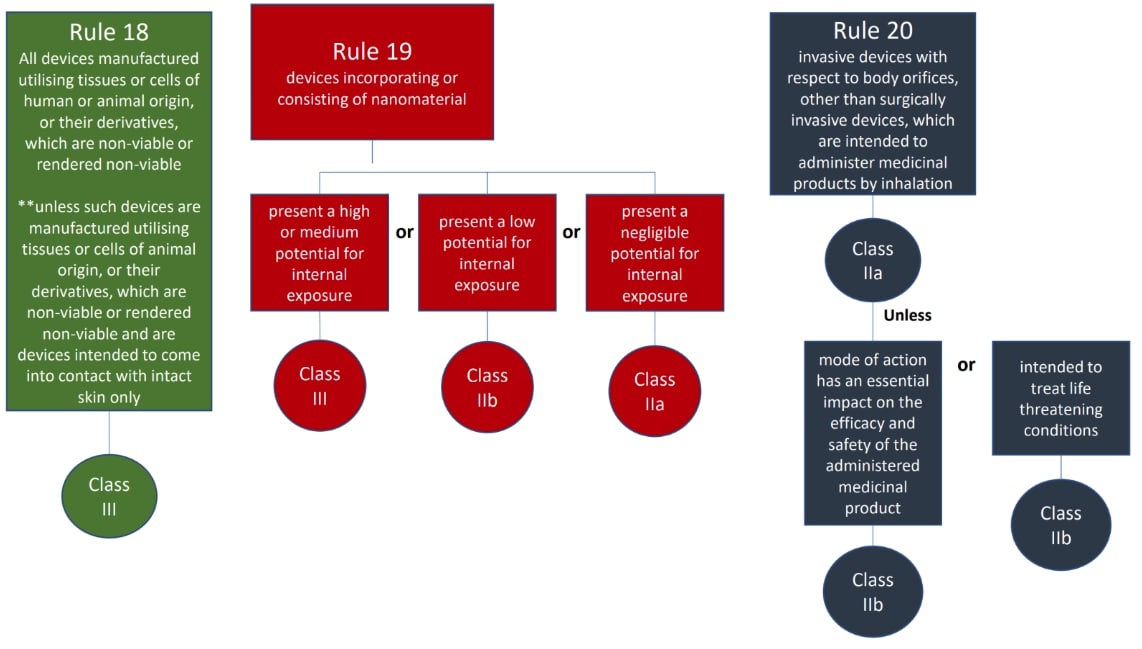
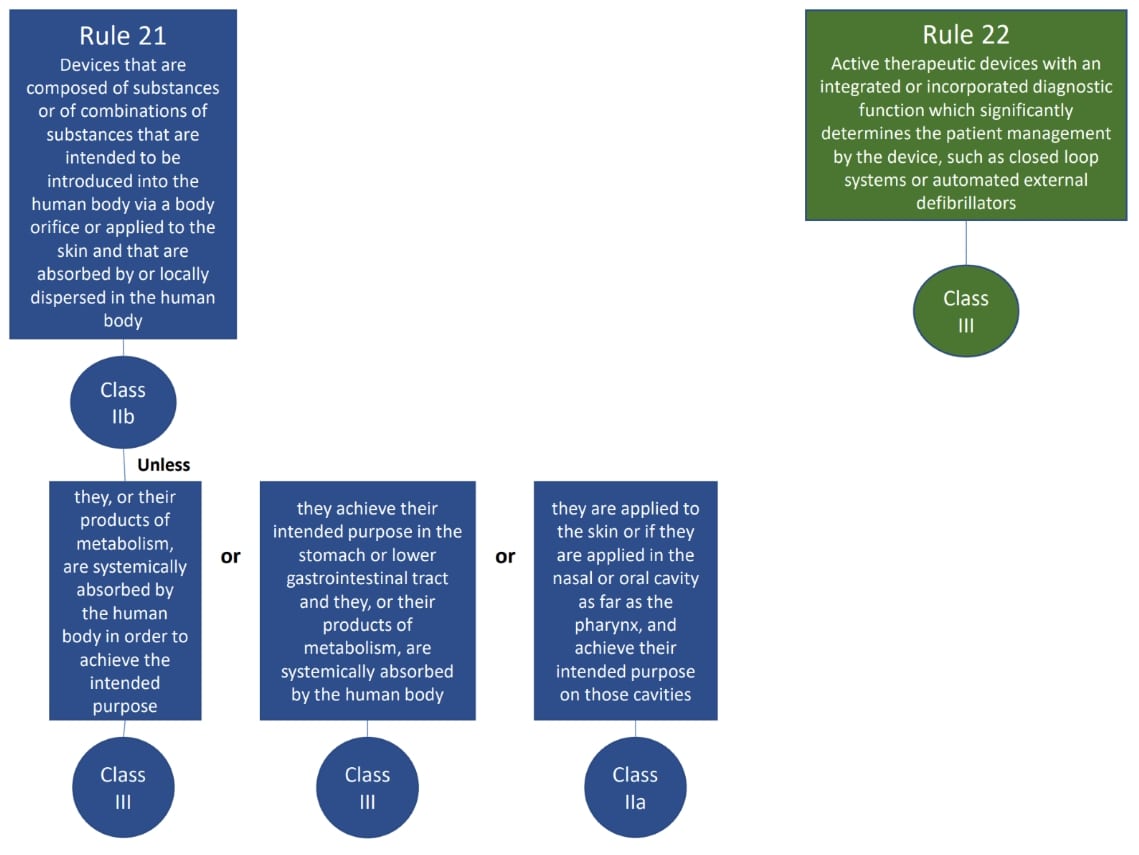
We hope this has been a useful guide on where to start with your classification, if you have further questions, please do review MDCG 2021-24, it also contains key definitions and the implications of the classification.

