PMCF is considered under the regulations to be a continuous process that has two critical functions – Firstly it is an essential part of clinical evaluation, and secondly it is a regulatory obligation as part of the post market surveillance system that a manufacturer must have in place.
WHAT IS IT INTENDED TO DO?
To confirm the safety and performance throughout the expected lifetime of the device
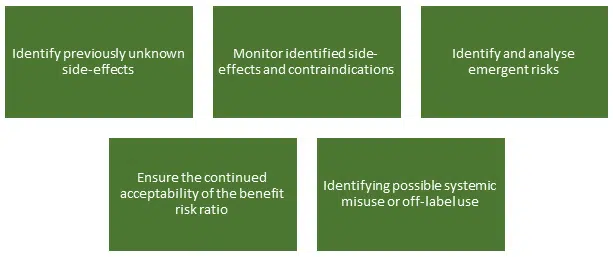
The new requirements under the EU MDR 2017/745 reinforce the importance of PMCF processes, with part B of Annex 14 being given to explaining the requirements. . Post market clinical follow-up is a general obligation of manufacturers and is always applicable as detailed under Article 10(3), and is not to be confused with a PMCF study/investigation or specific PMCF studies.
This blog will focus on the PMCF Plan and the specific requirements around this requirement.
REGULATORY REQUIREMENTS:
The MDR is very specific on the PMCF being part of the quality management system and part of the technical file included in the clinical evaluation plan and PMS plan.
Annex XIV Part B |
6.2. The PMCF plan shall include at least: |
1- the general methods and procedures of the PMCF to be applied, such as gathering of clinical experience gained, feedback from users, screening of scientific literature and of other sources of clinical data; |
2- the specific methods and procedures of PMCF to be applied, such as evaluation of suitable registers or PMCF studies; |
3- a rationale for the appropriateness of the methods and procedures referred to in points (a) and (b); |
4- a reference to the relevant parts of the clinical evaluation report referred to in Section 4 and to the risk management referred to in Section 3 of Annex I; |
5- the specific objectives to be addressed by the PMCF; |
6- an evaluation of the clinical data relating to equivalent or similar devices; |
7- reference to any relevant CS, harmonised standards when used by the manufacturer, and relevant guidance on PMCF; and |
8- a detailed and adequately justified time schedule for PMCF activities (e.g. analysis of PMCF data and reporting) to be undertaken by the manufacturer. |
The PMCF should be systematic generation, identification, appraisal and analysis of the longer-term data relating to performance and safety of the device, it is essential to ensuring continued compliance to the GSPRs. As required by Part B Annex XIV and Annex III the plan needs to include all the relevant information to conduct the PMCF.
MDCG 2020-07 POST-MARKET CLINICAL FOLLOW-UP (PMCF) PLAN TEMPLATE: A GUIDE FOR MANUFACTURERS AND NOTIFIED BODIES
MDCG 2020-07 aims to help manufacturers in meeting the requirements set out above. The template also seeks to achieve a ‘harmonised and complete presentation of post market clinical data and facilitate the activity of notified bodies and competent authorities in finding the information’.
WHAT DOES THE TEMPLATE REQUIRE?
Section A
Manufacturer contact details
Section B
Medical device description and specification.
This includes intended purpose, intended users, Basic UDI-DI, intended population, indications, contraindications and warnings. As well as accessories, certificate number, classification rule etc.
Section C
Covers the Activities planned for PMCF. It is down to the manufacturer to plan a PMCF that will provide the data needed to demonstrate the continued compliance already mentioned. The selection of activities should be appropriate and justified, there is not one solution for all. The MDCG template sets out detailed guidance on the detail required for this section. The high level sections are noted below
- Define where the need of conducting the PMCF activity is coming from (requested by notified body, clinical evaluation report, PMS, risk management report, previous PMCF report, etc.)
- Provide the description of each activity, and if it is a general or specific procedure / method.
- Define the aim of each activity
- Describe the different procedures which will be used as part of PMCF
- Describe the rationale for the appropriateness of the chosen methods/procedures
- Provide the timelines of the activity
A summary table template is also provided.
Section D
Reference to the relevant parts of the technical documentation. Where appropriate there should be a cross reference to the Clinical Evaluation Report and risk management File.
Section E
Evaluation of clinical data relating to equivalent or similar devices, in this section manufacturer shall document information regarding equivalent/similar devices for which clinical data will be further evaluated and presented in the PMCF report.
Section F
Reference to any applicable common specifications, harmonised standards or applicable guidance
Section G
Estimated data for the PMCF evaluation report
The full template and guidance can be found here
The team at Eclevar MEDTECH can support you in reviewing your PMCF needs, review your plan and help you ensure you have a sound and robust system in place to generate the data needed to support your device.

