There are many new challenges for manufacturers under the EU MDR and EU IVDR, one particular one is the identification of a Person Responsible for Regulatory Compliance (PRRC) – conveniently set out in Article 15 of both regulations!
This blog aims to be a quick reference guide to those requirements.
WHO SHOULD THE PRRC BE?
Under the new requirements manufacturers and authorised representative shall identify at least one person to be permanently and continuously available who is responsible for regulatory compliance.
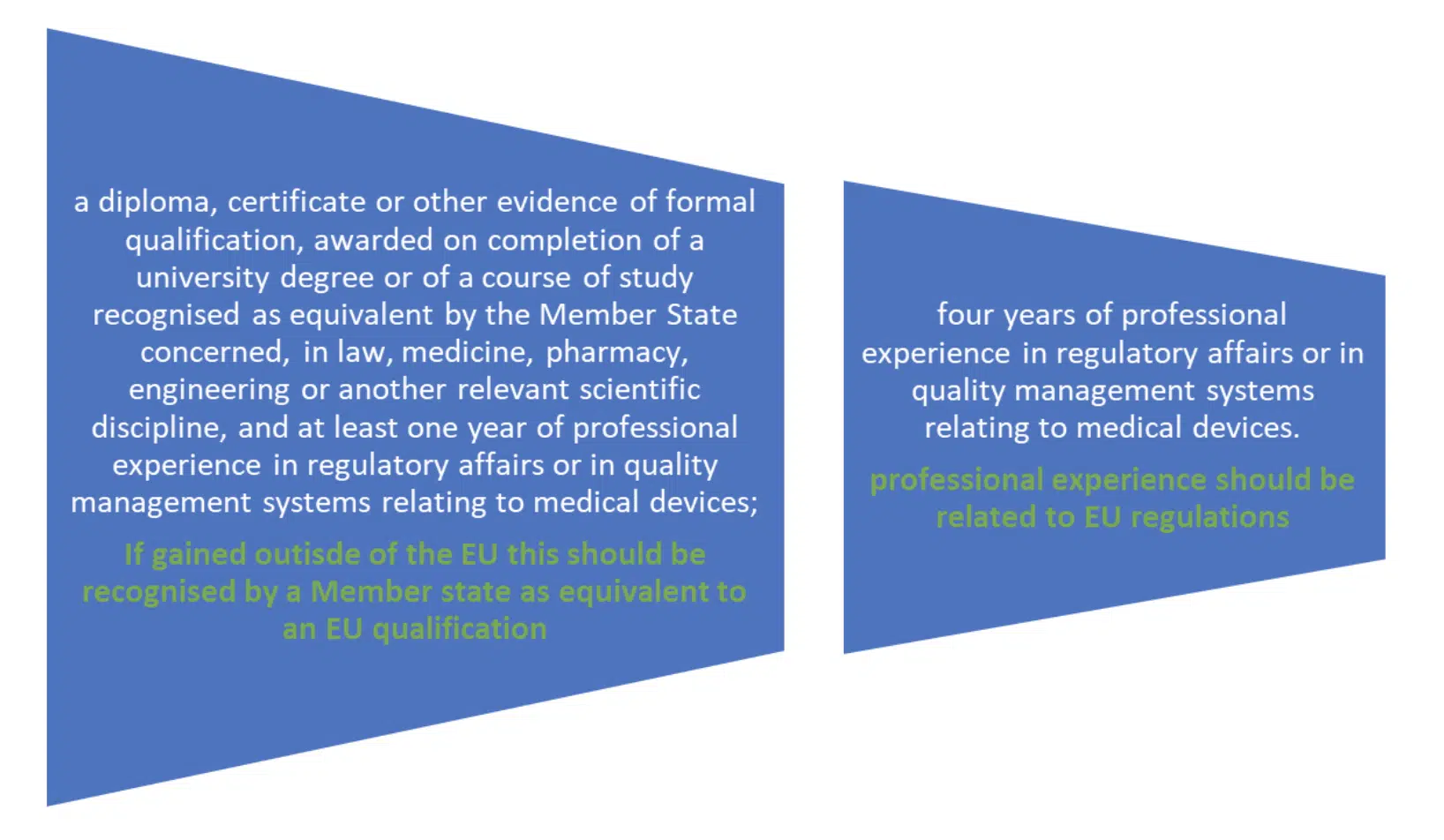
The required qualifications are also set out the PRRC must meet either of the following:
‘within their organisation’ – this is only applicable to medium/large companies, for these companies a PRRC is expected to be an employee of the company.
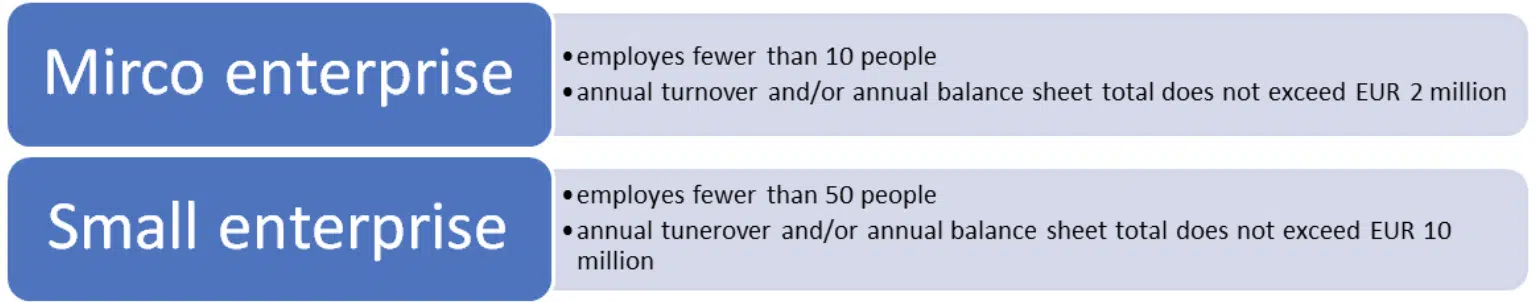
Micro and small enterprises are allowed to subcontract this role to an external supplier. The definition of such enterprises is set out in Commission Recommendation 2003/361/EC – Annex 1:
Whilst they do not need to be an employee of the company – they do still need to be and permanent and continuous disposal of the manufacturer.
Authorised Representatives must have a PRRC that meets the above qualification criteria, however they are able to subcontract the responsibility to a third party. Again, this must be at least one identified person and that person must be permanently and continuously at the Authorised Representatives disposal.
Where the PRRC is subcontracted – the contract should clearly set out the relevant qualifications and the set out the agreement regarding that person(s) role and requirement for them to be at the company’s disposal as required in the Regulations.
Where should the PRRC be based?
- For manufacturers based in the EU – the PRRC will be also based in the EU
- For manufacturers based outside of the EU – the PRRC will be based outside of the EU.
- For Authorised Representative the PRRC will be in the EU.
WHAT DO THEY HAVE TO DO?
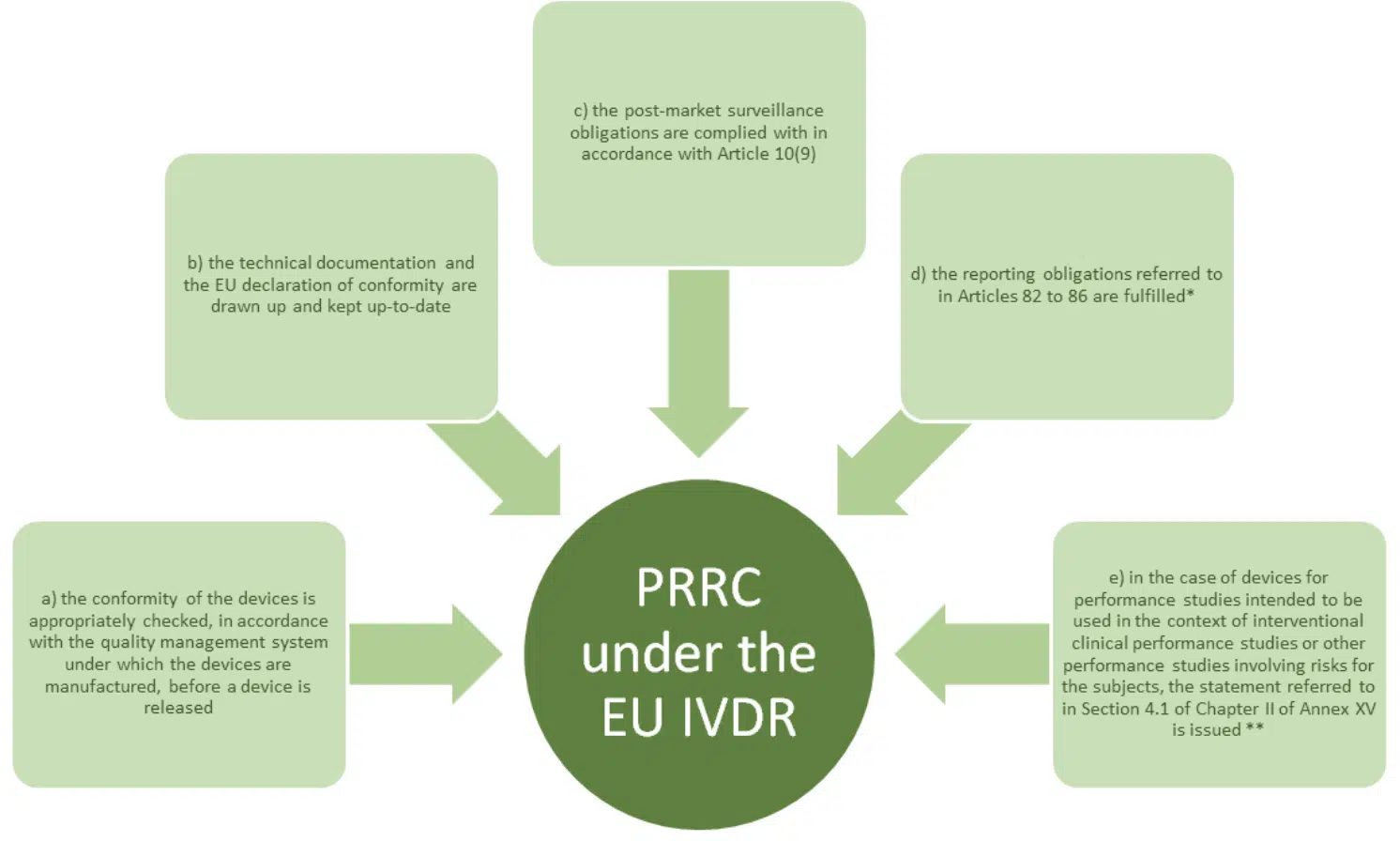
Article 15 (3) lists the key functions of the PRRC. In addition to the regulations themselves, MDCG 2019-7 provides a cross reference to the obligation of a manufacturer and AR respectively, this provides useful additional context and should be read!
Note that if you choose to have more than 1 PRRC, they can share the role and responsibilities – any split of the responsibilities should be clearly agreed and documented in writing.
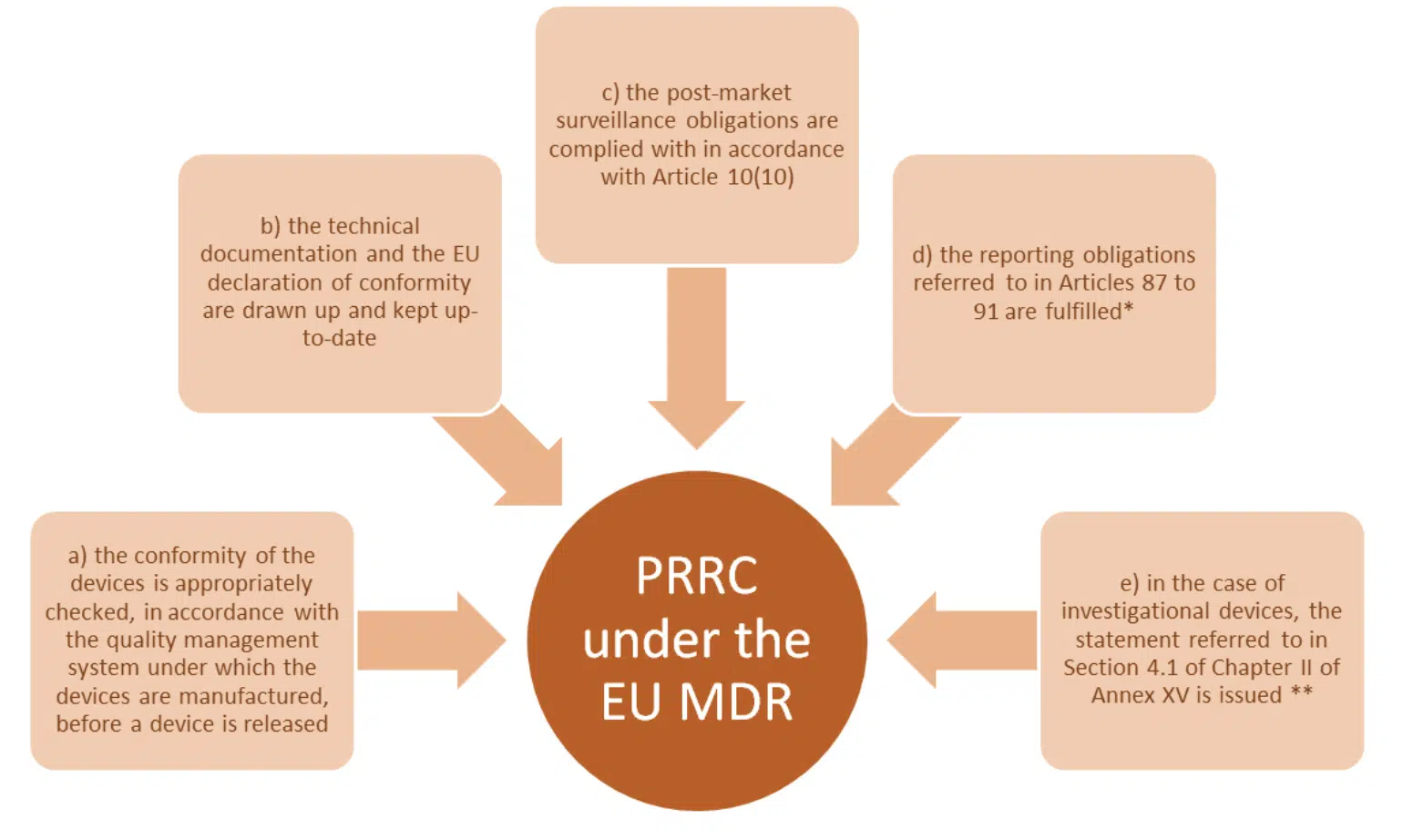
Note: * refers to vigilance requirements and ** relates to GSPR conformity.
WHY DO YOU NEED A PRRC?
In short, this is to add clear responsibility for monitoring compliance for key activities. The requirements set out to ensure that these key functions are conducted by a person or persons with relevant qualifications or experience in the field.
This seeks to provide that increased scrutiny around compliance to the requirements – a ‘golden thread’ that runs through both regulations.
Once in place and responsibilities are set out and agreed, this role should actually assist companies in maintaining compliance with their obligations.
Relevant reading:
- EU MDR Article 15
- EU IVDR Article 15
- MDCG 2019-7 Guidance on Article 15 of the Medical Device Regulation (MDR) and in vitro Diagnostic Device Regulation (IVDR) regarding a ‘person responsible for regulatory compliance’ (PRRC)
We hope this has helped set out the requirements and considerations when putting one in place. For companies already on the EU market the PRRC should be in place already but do make sure the requirements are being met by your arrangements.
If you have any further questions do get in touch….