Understanding the Transition from IVDD to IVDR and UK MDR 2002: Key Takeaways
The transition from the EU In Vitro Diagnostic Devices Directive (IVDD) to the In Vitro Diagnostic Regulation (IVDR) and the UK MDR 2002, particularly regarding the use of the CE mark for accessing the GB market, presents several challenges. Based on the available information and infographic, here are some key elements to better understand the process:
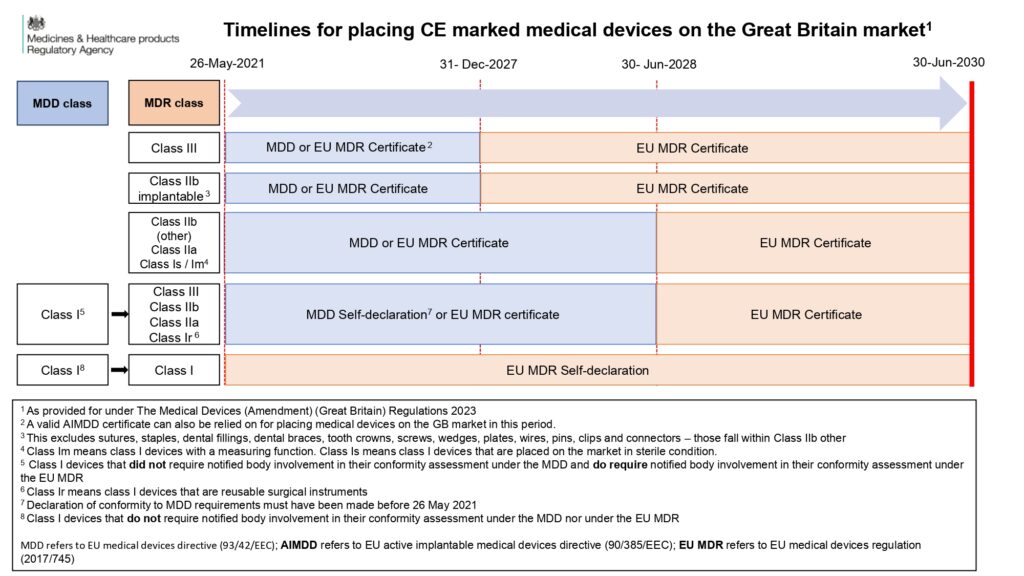
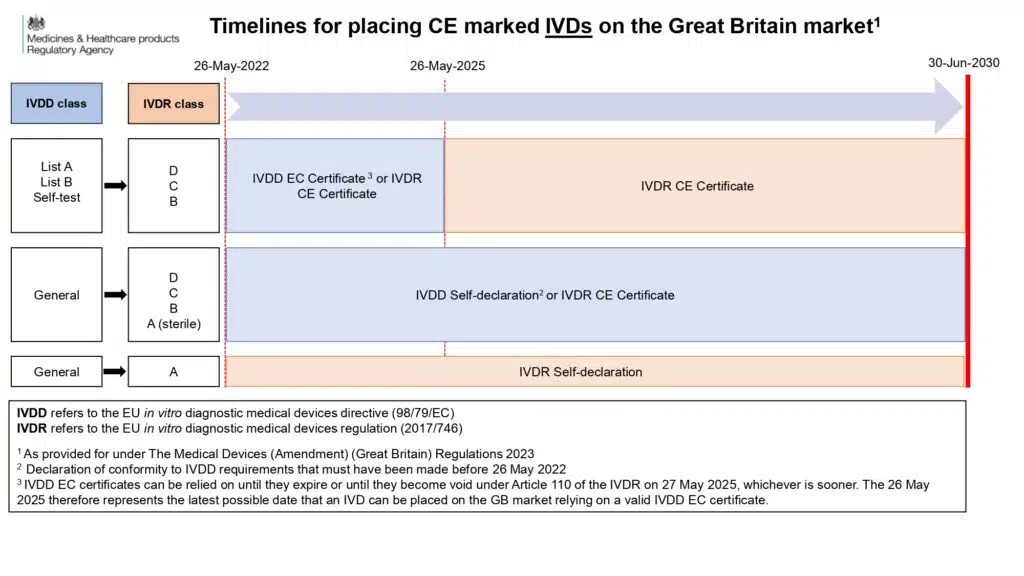
1. ‘General IVDs’ Under IVDD, Now Up-Classified Under IVDR:
- Manufacturers who self-declare their devices to the EU IVDD, with a signed Declaration of Conformity by 26 May 2022, can use this documentation for accessing the GB market as long as the provisions of IVDR Article 110 allow. Therefore, it is essential for manufacturers to also refer to IVDR Article 110.
- If a device is certified under the IVDR, the IVDR CE mark can be used until 30 June 2030 to access the GB market.
- The information presented in the infographic solely pertains to using CE marking for GB market access. It’s important to note that ‘UKCA marking’ is currently possible. For general IVDs under the UK MDR 2002, manufacturers can self-declare against the UK MDR 2002 until 2030, provided the Declaration of Conformity is signed before the expected effective date of the new UK MDR 2002 (1 July 2025). After 1 July 2025, the new UK legislation is expected to apply to new devices, but the previously signed Declaration of Conformity can still be relied upon until 1 June 2030 (based on the current understanding).
2. IVDs Requiring Notified Body (NB) Certification Under IVDD:
- Certificates issued by a Notified Body under the IVDD, including HIV tests, blood glucose meters, and self-test devices, will have an expiry date of either 26 May 2025 or earlier. It’s important to note that ‘renewal’ of IVDD certificates is not possible. Therefore, devices must be certified under the IVDR before their EC certificate expires. The same principle applies for accessing the GB market using the CE mark.
- Manufacturers can currently apply for a ‘UKCA mark’ for these devices from an In Vitro Diagnostic (IVD) Approved Body, in accordance with the current UK MDR 2002. This certificate is expected to remain valid beyond 26 May 2025. We are awaiting further information on the transition timeline from the old to the new UK MDR 2002 for these devices, following the effective date of the new UK legislation.
3. Self-Declared Devices Under IVDD and IVDR (Class A Non-Sterile):
- Class A non-sterile devices, such as instruments, should comply with the IVDR from 26 May 2022. Manufacturers must self-declare their devices against the IVDR for those placed on the market after 26 May 2022. The IVDR CE mark can be used for accessing the GB market until 30 June 2030.
- Manufacturers also have the option to self-certify against the UK MDR 2002, enabling continued access to the GB market. It’s crucial for manufacturers to stay updated on any new provisions introduced when the new UK MDR 2002 is published.
Please keep in mind that the information provided here is based on the current understanding, and it’s important to stay informed as more details and clarifications emerge