UKCA Mark: PMS & PMCF – what changes are proposed for the UK?
In looking to achieve more harmonization across the industry and improve regulatory oversight of the post-market space, the MHRA have set out their proposal to have extensive and clearer requirements.
WHAT DOES THE CONSULTATION RESPONSE TELL US?
In general, what we see is a more proactive approach, as seen with the introduction of the EU MDR & IVDR. MHRA say they seek to ‘improve patient safety and strengthen the level of post-market surveillance activities conducted across all manufacturers placing… on the UK market’
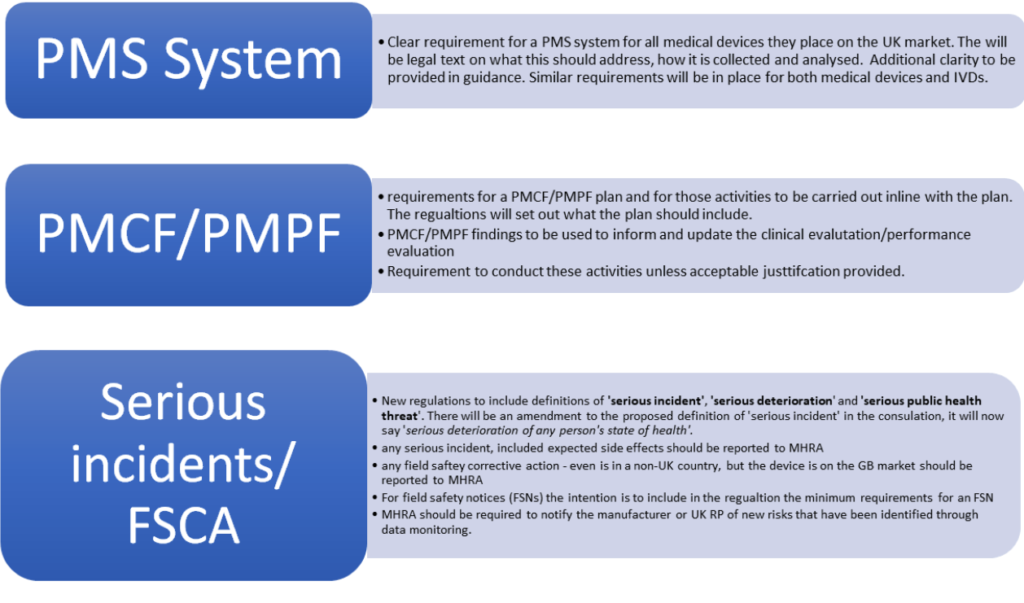
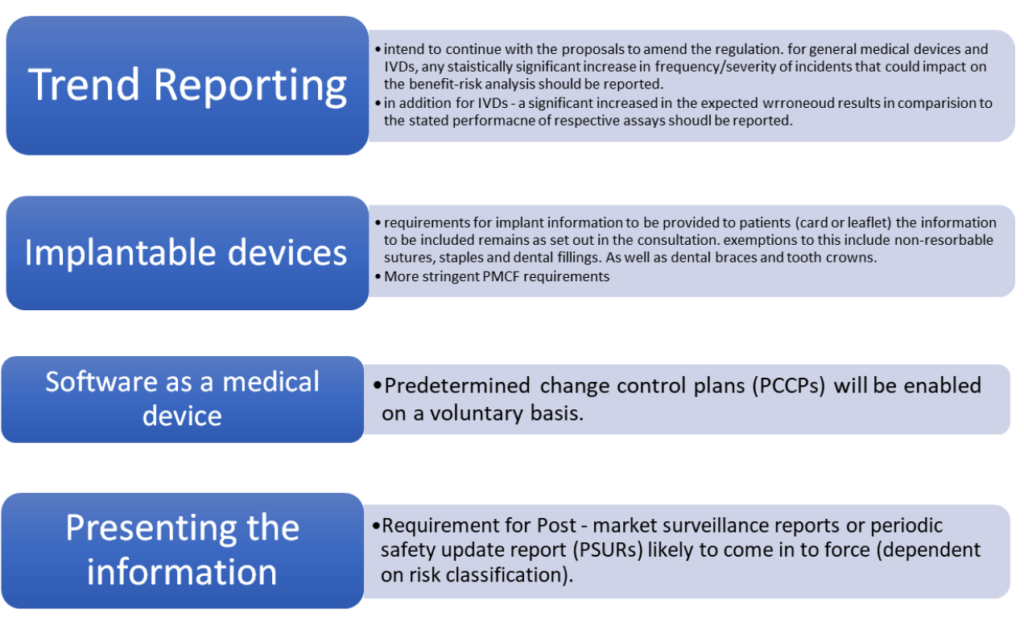
Set out above are the points that the government intended to proceed with, there are points that MHRA will consider further. For example, in the case of FSN submissions for comment – they are awaiting appropriate systems to be in place.
WHAT’S DIFFERENT BETWEEN THE EU MDR & IVDR?
Not a lot! There are significant synergies between the consultation response and the new EU regulations.
However, there are variations on the theme and some additions such as PCCPs, potentially more robust requirements around timelines of PMPF, slight variations on definition, etc.
Much of the consultation response, states that the variations seek to provide clarity, build a more robust system, and to further protect patient safety.
WHAT’S THE IMPACT ON THE MEDTECH AND IVD SECTOR?
The extent of the synergies should hopefully be welcomed, this should enable minimal duplication of effort in terms of the data generation and assessment.
Where the risk of ‘burden’ comes is in the processes. It is hoped the MHRA will continue to be pragmatic and ensure they have the systems in place that ease the process and ensure that sound post-market data can be reported and supplied as necessary. Clear and timely guidance is key and hopefully, further information will be provided soon.
The requirement for MHRA to inform manufacturers or UKRPs around new signals/risk is a real opportunity – it opens the door to more collaborative working based on a more extensive data set. If successful, this should further improve patient and public safety.
This blog has looked to pull out some of the key proposals from the consultation response, of course there is much more detail and consideration to be had. If you would like to discuss the UK requirements now or the impact of the future plans, please contact us.

