It is fair to say that the MedTech sector breathed a sigh of relief when MHRA published the consultation response on 26th June 2022.
It is a very considered, ambitious and pragmatic response, there are areas still not fully addressed, but over all a very positive response to a consultation that had seen nearly 900 responses received by MHRA. What we see is a proposal that is largely aligned to the principles set out in Regulation (EU) 2017/745 & Regulation (EU) 2017/746. However, what is critical is the UK have the ability to focus on the UK alone and what is right for UK patients and the MedTech sector.
The have 5 ambitious themes, working to ensure that patients and the public are at the core of all they do. MHRA are seeking to ensure patients and the public are protected and have access to the newest advances in the MedTech sector.
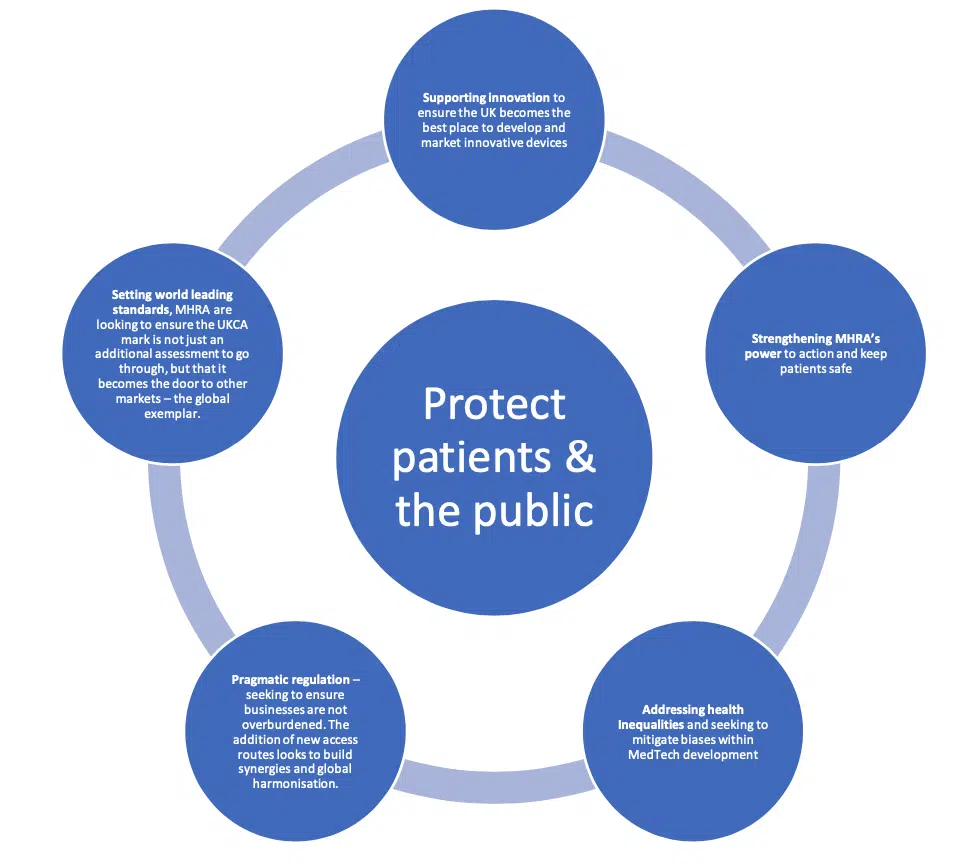
There is an awful lot in the 155 pages to process, and there are of course areas for clarification. We will seek to keep you up to date on any further information or clarification.
There are many areas that warrant further discussion, however in this piece we will focus on
TRANSITION.
What does the response tell us?
MHRA confirm that there will be a transition period. Great! But what does that mean for industry?
Market Access
UKCA Marked
• Applies to general medical devices and IVDs that hold a valid UKCA certificate/DoC before the new regime takes full effect
• Allow products to be placed on the market until either the existing certificate expires or for 3 years (general MDs) or 5 years (IVDs) after the new UK regualtions take effect, whichever is sooner.
• Caveats:
• devices are subject to significant changes in design or intended purpose will be excluded
from these provisions
• All post-market requirements applicable to the new framework will need to be complied
with for products using the tranitional arrangements
MDD/IVDD CE marked
Applies to general medical devices and IVDs that hold a valid MDD/IVDD/AIMDD CE certificate/DoC before the new regime takes full effect
Allow products to be allowed on the UK market until either the existing certificate expires or for 3 years (general MDs) or 5 years (IVDs) after the new UK regualtions take effect, whichever is sooner.**
Caveats:
• devices are subject to significant changes in design or intended purpose will be excludedfrom these provisions
• all post-market requirements applicable to the new framework will need to be compliedwith for products using the transitional arrangements.
EU MDR/IVDR marked
• Applied to general medical devices and IVDs that hold a valid EU MDR or IVDR CE certificate/DoC.
• Allow products to continue to be placed on the market until the existing certificate expires or for 5 years after the regualtions take effect, whatever is sooner. **
•Thiswillapplyevenifthecertificate/DoCisdatedafterthenew UKregulationstake effect. However to note the 5 years period remains from when the UK regualtions take effect.
MHRA will review at the 5 year point.
Caveats:
• devices are subject to significant changes in design or intended purpose will be excluded from these provisions
• all post-market requirements applicable to the new framework will need to be complied with for products using the transitional arrangements.
**The proposed requirement that CE marked products (either under the Directives or Regulations) would have to be registered with MHRA, and for that application to be completed before the new regulation take full effect, will not be taken forward.
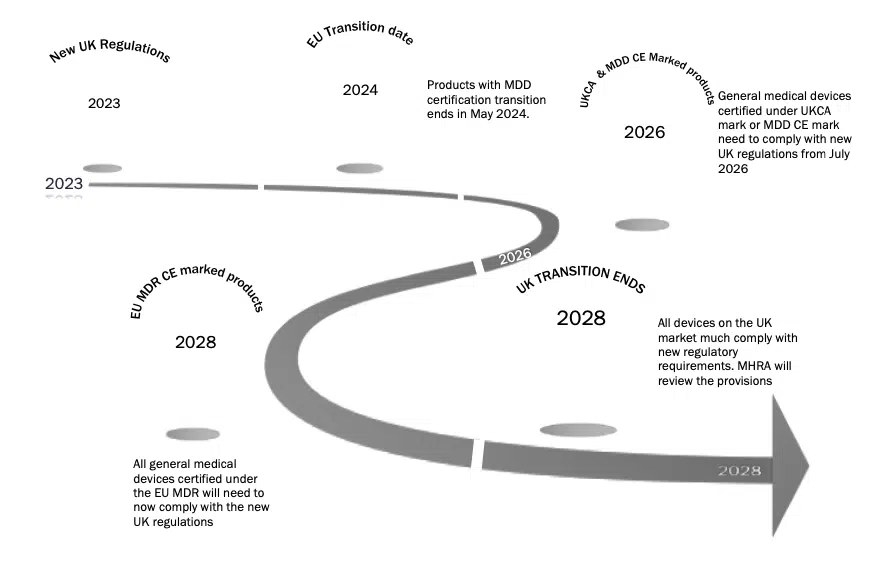
Below we have provided provisional timelines based on the provisions set out, it is important to note that this has been based on the date of 1st July 2023 as was proposed in the consultation document:
General medical devices
IVDs
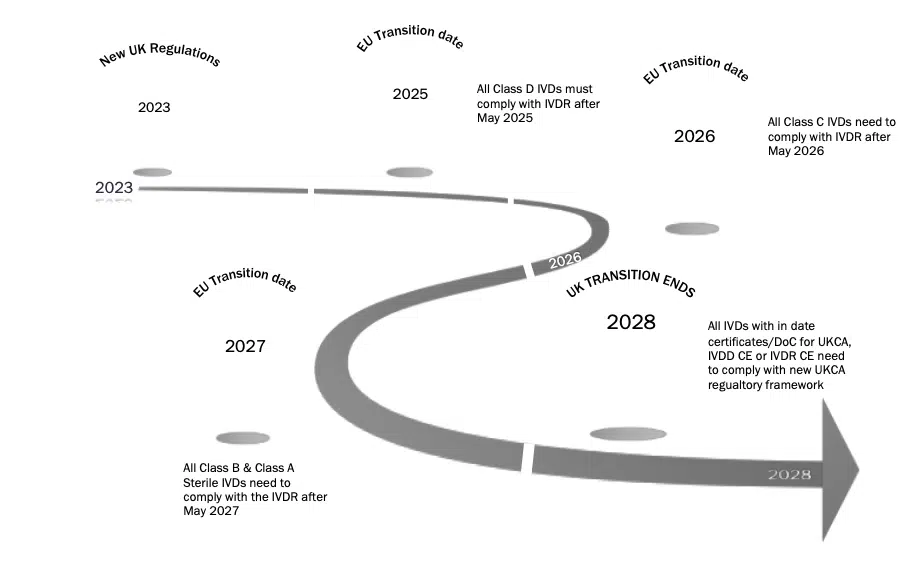
To note: the use of the term ‘full effect’ is important – once the statutory instrument is laid, there is a 6-month period before the regulations can be enforced. This is part of the obligations of the TCA – article 94 (8). Clarification is required on when the regulations will be laid and therefore when they will come in to full effect.
Clinical Investigations & Performance Studies
Clinical Investigations
•Applies to clinical investigations that commence under the current regulations and which would not have completed before the new regualtions take effect.
•No requirement to re-apply to MHRA
•The ongoing clinical investigation must comply with all
reporting requirements set out in the new regulations once they come in to effect.
Performance Studies
•No transition provisions
•Significant changes in the regulation on performance studies and evalution.
There is a need for clarification on dates, but overall this is a welcome step to ensure that there is not a supply and research ‘cliff edge’ when the new regulations come in to force.
A smooth implementation is key, and there are lessons to be learnt from the implementation of the EU MDR & IVDR. Timely guidance, transparency and collaboration across the sector are vital. The MHRA had committed to focus groups to support the implementation, a very welcome step, these will be critical in ensuing the implementation is achievable and the sector has the information it requires to transition accordingly. However, system readiness is still a concern particularly around system capacity to achieve this transition dates.

