ECLEVAR MEDTECH is positioned with unique access to a world-renowned Vascular lab and angiography core laboratory and a clinical research site actively participating in over 30 cardiovascular trials:






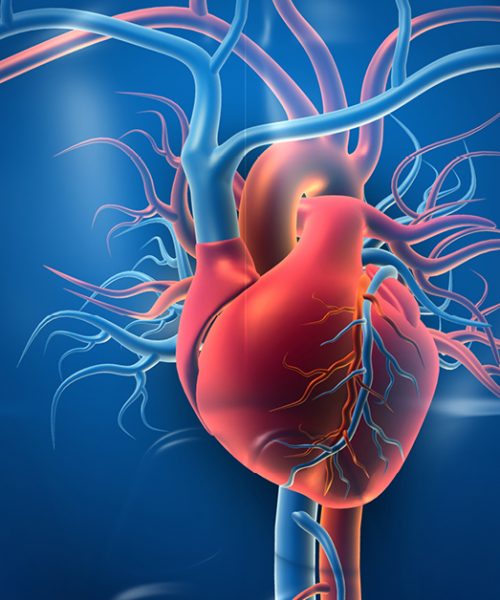
A vascular medical device can be defined as a device that is used during open surgery techniques, as well as endovascular techniques to treat control, diagnose, monitor, or correct disease or injury. It encompasses the cardiovascular, peripheral vascular, and neurovascular anatomy.
The Vascular team specializes in implant, endovascular prothesis stents intended to be used in the arteries and veins for the diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease or injury, or in the investigation, replacement or modification of the anatomy in relation to the vasculature or the heart.
We collaborate with our in-house clinicians and technical teams specialized in endovascular surgery and clinical investigation.
Examples of products we cover include:





ECLEVAR MEDTECH will provide the following DSMB services:
DSMB Setup Activities:
Per Meeting Activities:
Data Safety and Monitoring Boards (DSMBs), also called Data Monitoring Committees (DMCs), were introduced in first time in 1960s to ensure the safety of patient in clinical investigation. The main difference between DSMBs and other clinical oversight bodies is that a DSMB undertakes periodic risk-benefit evaluation during the medical device clinical investigation using the clinical data gathered during the study to oversight the emergence of serious or unexpected adverse outcomes.
The DSMB shall request stopping a trial for evidence of harm. For less serious adverse events, the DSMB may serve as a conduit for relevant information to the medical device sponsor that can trigger protocol amendments, changes in surveillance or further training of study investigators.
CEC Setup Activities:
Per Meeting Activities:

Cardiovascular medicine is increasingly dictated and regulated by evidence-based guidelines stemming from randomized trials and registries. The ECLEVAR MEDTECH Research Center for Advanced Imaging and Core laboratory provides cutting-edge imaging methods and decades of cardiovascular imaging expertise to assess the imaging component of these trials and registries to ensure they are reproducible and yield the best outcomes.
Facilities include the following:


Each step is handled by our team of experts (Biostatisticians, Data Analysts, Clinical Research Assistants, Clinical Project Managers) who rely on best clinical practices such as ISO14155:2020, advanced analysis techniques and a strong Quality Management System.

With ECLEVAR MEDTECH, a feasibility study is conducted before engaging in RWE clinical study to ensure the quality and quantity of data. It is important to demonstrate that the data source is of sufficient quality and quantity for the intended use. The advantages of using RWD as a valid PMCF activity are of no relevance if the data source is of poor quality, as reliable decisions cannot be made based upon an unreliable data source.
The medical device:
vascular prostheses made of knitted polyester fabrics, in straight tubular shapes, impregnated with ultra-purified collagen of bovine origin, and are indicated for replacement or bypass of arteries damaged by an aneurysm or arterial occlusive disease.
Problem:
As shown by the Clinical Evaluation Report, intended claims on clinical safety and performance are not sufficiently supported by existing clinical evidence. To maintain a “Vascular patch “in the European market, the sponsor has to conduct a PMCF study to generate sufficient clinical data in accordance with “Chapter VI – Clinical Evaluation and Clinical Investigations, specifically sections 62 – 82”. As well as ISO 14155:2020.
Objectives
Methodology
A Sponsor conducted RWE multicentre study to collect clinical data for Vascular patch. The objective was to examine short and long-term outcomes of using the device when exposed to a larger and more varied population.
All data were retrieved from medical charts for each patient from the time of surgery (considered as the baseline of study) until a maximum of 3 years after surgery.
A minimum of 250 up to a maximum of 300 subjects were evaluated from 3 to 8 different sites. At least 100 subjects were evaluated in carotid location and at least 100 in femoral location.
Hospital network involved:
Our KOLs Involved in the study:
Benefits
Applicable regulation and guideline:
We are a global organization, trusted and recognized around the world. ECLEVAR MEDTECH can support clinical trials in EU, UK, Australia and Japan.
We are equipped with cutting-edge technology in the vascular area in collaboration with CORRIB Lab.
The benefits of having experienced, professional and well-qualified technical specialists cannot be overstated in the complex and ever-changing medical device industry. Our Clinical project managers are specialized and experienced with Vascular clinical trials.

As vascular medical device CRO, we have successfully conducted complex vascular projects. See clinical trial.gov below: -Vascular Stent -Endovascular protheses

Subscribe to our newsletter

VISIT US
ECLEVAR FRANCE:
231 rue Saint-Honoré, 75001 Paris, France
ECLEVAR GMBH
ERFURT, Erfurt Hauptbahnhof
4th, 5th floor
Bahnhofstr. 38 Erfurt 99084
ECLEVAR Australia
Umina Beach NSW 2257, Australia
ECLEVAR UK Limited
3rd Floor 207 Regent Street, London, W1B 3HH
CONTACT US