Different regulatory jurisdictions utilise ‘equivalence’ in different ways, from forming part of a clinical evaluation to offering a direct route to market. We are going to consider its use across the globe, starting with Great Britain.
The current UK MDR 2002 is based on the three EU directives, this is applicable to England, Scotland & Wales. As with the EU Directives, the use of equivalence continues to be a valid method for data generation for a clinical evaluation. Whilst not part of the current legal framework, the UK regulator, MHRA, and the UK Approved Bodies consider compliance with the principles of MEDDEV 2.7/1 revision 4 to be critical.
As such, there are similarities in the expectations when compared to the EU MDR (stay tuned for the next in this series).
MEDDEV 2.7/1 revision 4 provides guidance for manufacturers and notified/approved bodies on clinical evaluation – this blog is focused on Appendix I and the use of equivalence.
When used in line with Appendix I of MEDDEV 2.7/1 rev.4, data from equivalent devices can be a scientifically valid approach to clinical evaluation. However, it is sometimes seen as a ‘shortcut’ in the process. We hope this blog will help you understand the steps and some of the errors made.
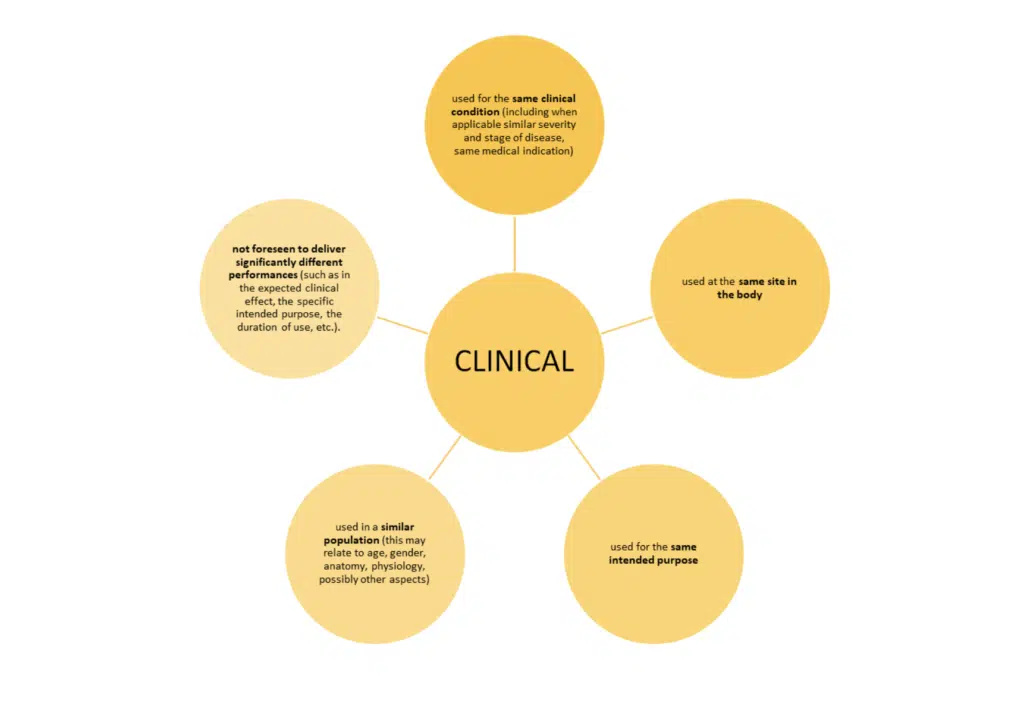
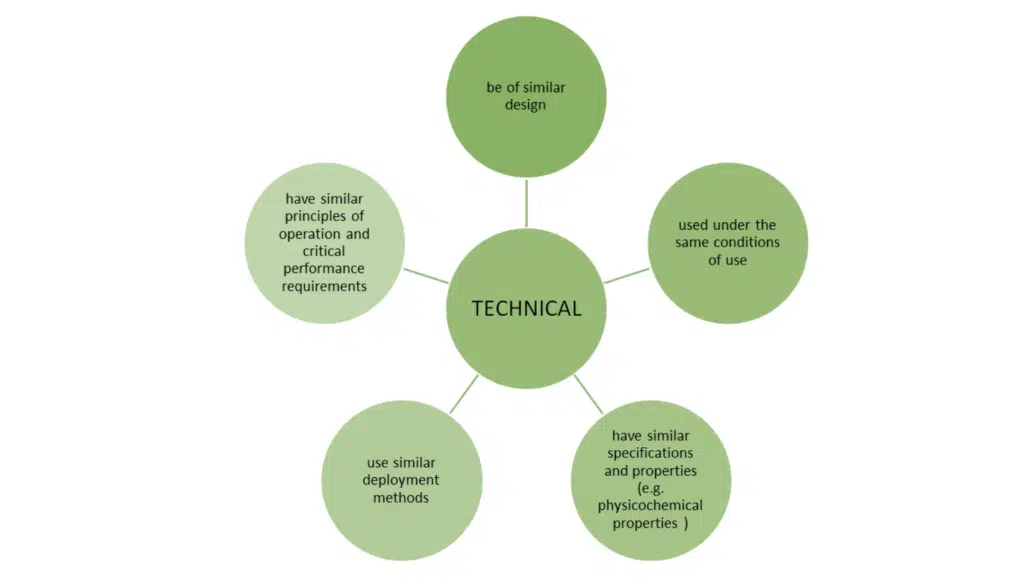
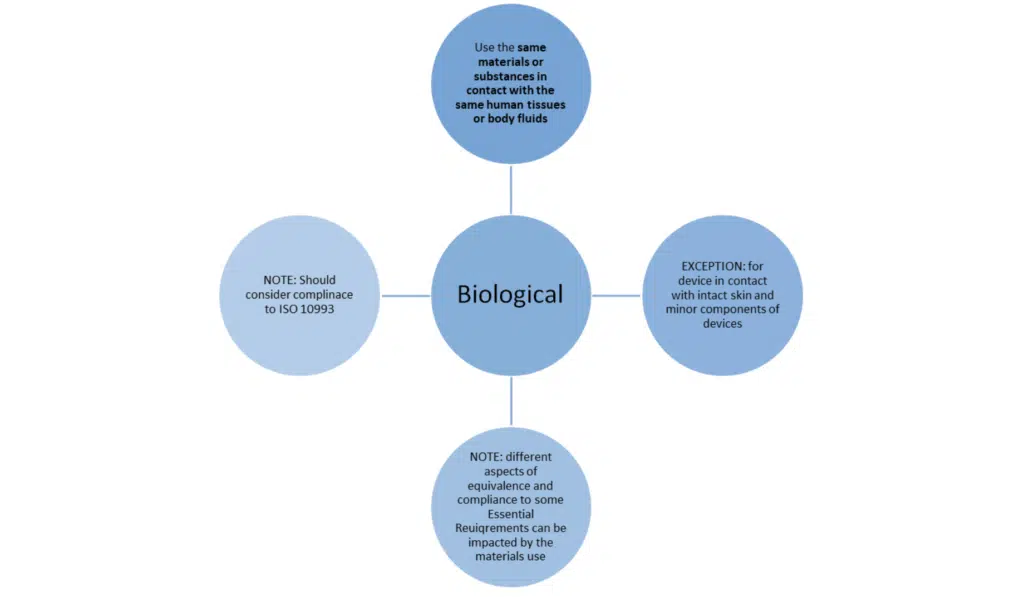
Just meeting these bullet points is not the whole story. MEDDEV 2.7/1 revision 4 sets out additional conditions when claiming equivalence to another device.
Attention to detail:
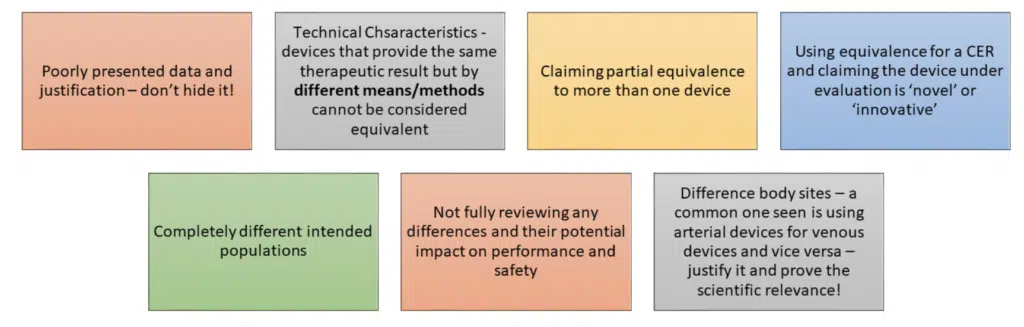
It is a scientific evaluation of data; it should be done thoroughly and used appropriately.
TAKE HOME MESSAGE:
If in doubt, read MEDDEV 2.7/1 revision 4 and ask an expert!
We hope this has helped set out the requirements and considerations when looking to use equivalence in your CER. For the detailed text, refer to MEDDEV 2.7/1 Revision 4 published in 2016.
If you have any further questions do get in touch.
Following on from our blog on equivalence in the UK, we will now turn our attention to the EU. Thankfully not a huge leap in terms of basic principles, but there are key requirements to be aware of when claiming equivalence.
The introduction of the EU MDR 2017/745 brought in the principles set out in MEDDEV 2.7/1 revision 4 in to the regulatory text, it was not unexpected, but some nuances of the text have created confusion and challenges for manufacturers.
There continues to be ongoing discussion around the interpretation of the regulatory text – however for the purpose of this blog we are following the general consensus and the further detail as set out by MDCG 2020-5 Clinical Evaluation – Equivalence A guide for manufacturers and notified bodies
Annex XIV Part A (3) sets out the characteristics that need to be met:
The use of the word ‘similar’ again means that the characteristics in question are similar to such an extent that there is no clinically significant difference in the safety and performance of the device as a result of the differences.
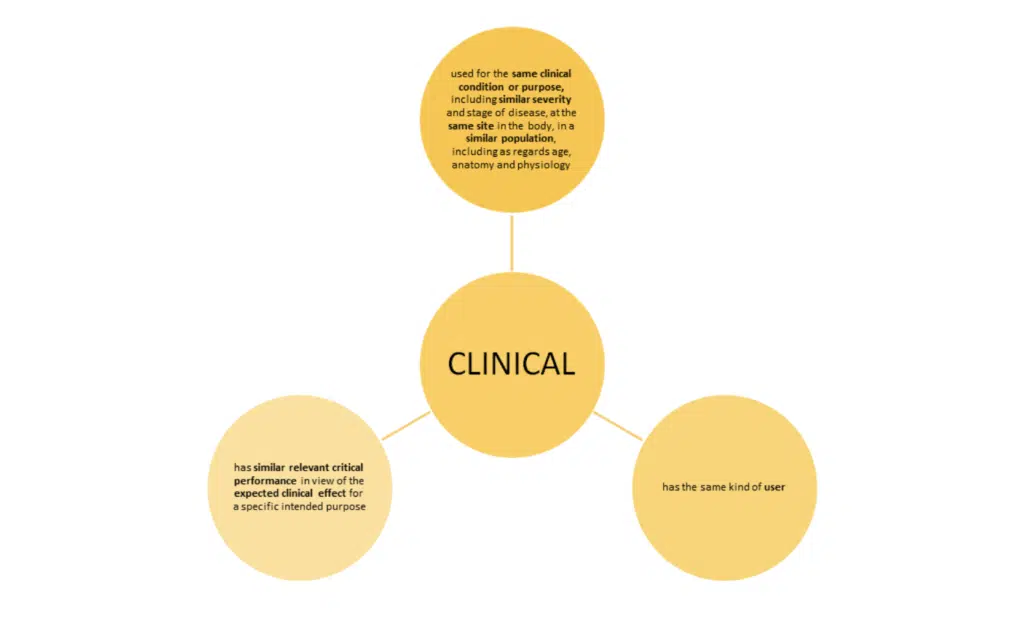
Note: ‘user’ means any healthcare professional or lay person who will use the device. It is important to consider the implication of the user’s level of competence on the safety and performance when considering equivalence.
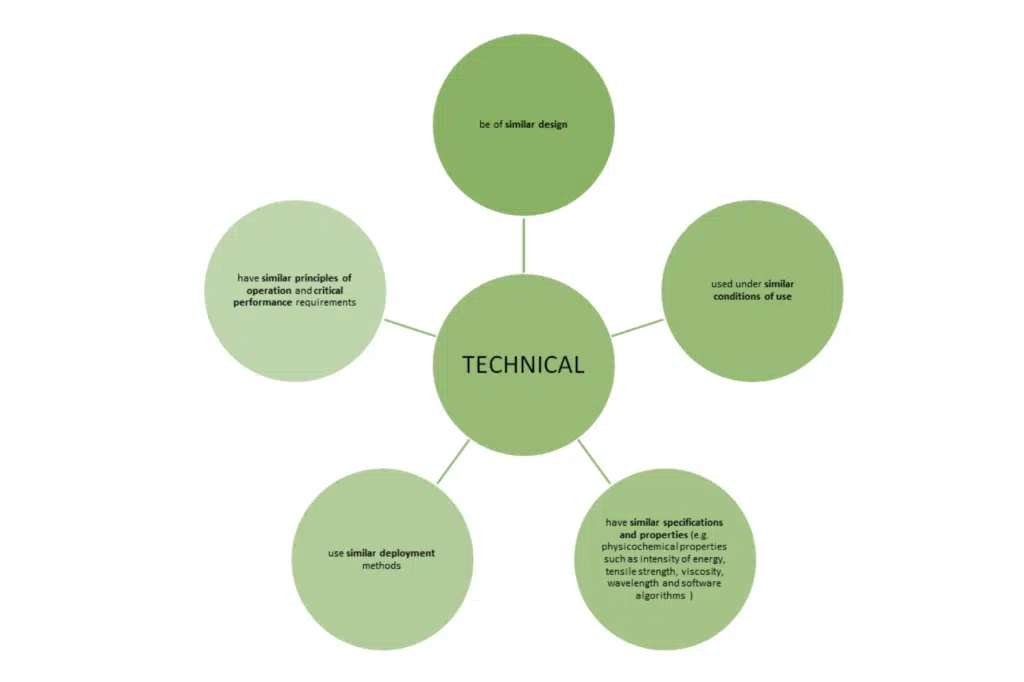
Note: the list of specifications and properties is not exhaustive, however important to note the clear inclusion of software algorithms in the MDR text. When reviewing the software algorithm, you should consider:
MDCG 2020-5 states that it is not reasonable to demand equivalence is demonstrated for the software code, as long as it have been developed in line with international standards for the design and validation of medical device software.
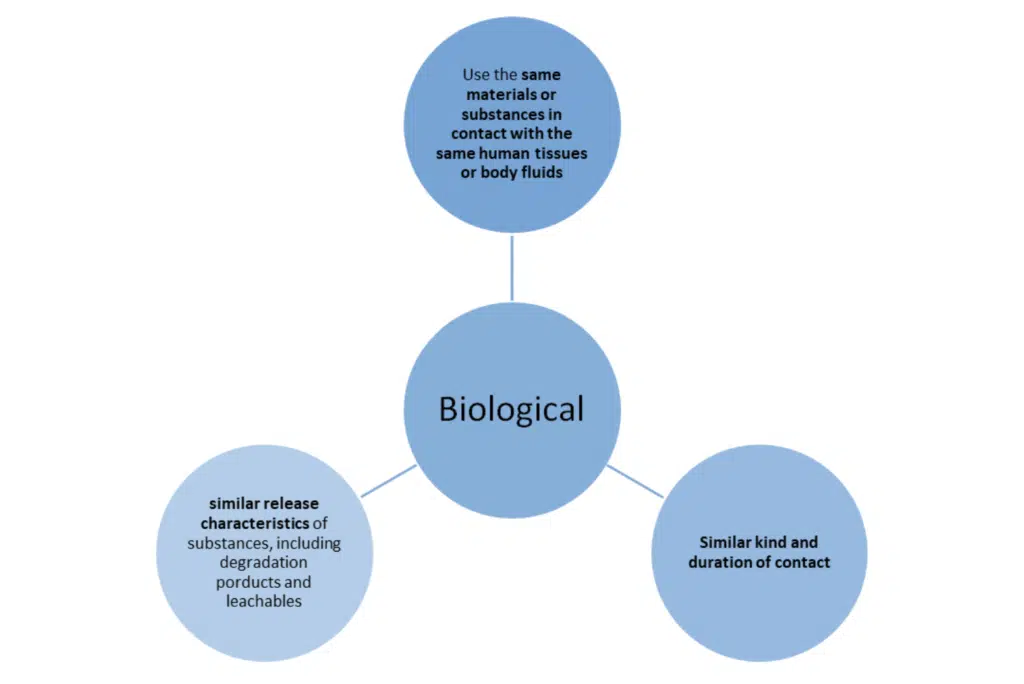
Note: in the exceptions previously set out in MEDDEV 27/1 revision 4 to not use the same material are not acceptable under the new regulations.
The ISO 10993 standards should be considered and can be adopted – some key sections to review:
Getting it right:
The MDR clarifies and adds in new requirements around the process of equivalence – below are some of the most important.
QMS – Annex IX, Chapter 1 2.2 (c) further tightens the regulations around equivalence, setting out the QMS requirements – manufacturers must set out their process for handling equivalence in their QMS.
Assessment & Gap analysis: Equivalence is part of the bigger clinical evaluation picture – manufacturers are required to specify and justify the level of clinical evidence that is necessary to demonstrate conformity with the general safety and performance requirements relevant to their device.
This must be based on robust scientific justification. With regard to equivalence this requires all three characteristics to be duly considered. All differences should be disclosed and evaluated.
Whilst some aspects of the characteristics must be the SAME, there are those that need only be SIMILAR, there must be a clear gap analysis performed and any differences evaluated.
Modifications: Modifications are common place however they do need to be considered carefully. MDCG sets out that if modifications are introduced to address a specific safety or performance concern, then the modifications are justified and that no additional clinically significant difference will occur.
More than one device? Unlike in MEDDEV 2.7/1 rev 4., the MDR allows for more than one equivalent device. If more than one device is to be used, then a full analysis of each product must be done and fully documented in the clinical evaluation report.
What should not be done is using different parts of different devices to pull together one equivalent device’. Although the MDCG guidance does set out that an exception could be made to this where a device has ‘stand-alone’ devices within it.
When seeking to claim equivalence for a medical device, a manufacturer cannot use a product without intended medical purpose (Annex XVI)
Implantables & Class III
Clinical investigations on the device under evaluation should be conducted for these risk groups – UNLESS the meet the conditions set out in Article 61 (4):
Equivalent device from another manufacturer? The conditions above need to be met but in addition the manufacturer must have a contract in place – allowing for full and ongoing access to the technical documentation of the equivalent device.
Devices other than implantables and class III: there is not stipulation that the equivalent device must have been assessed against the MDR – it is presumed devices marketed can be under the relevant Directive or the MDR. It may also be possible to claim equivalence to products without a CE mark – however the MDR requirements still must be met. The regulatory status of the product must be disclosed.
Whilst for these products a contract is not required, the manufacturer must still be able to demonstrate that they have sufficient access to the data that related to the equivalent device.
Devices composed of substances or of combinations of substances: where these substances are intended to be introduced into the human body, absorbed or locally dispersed in the human body – for the claim of equivalence, these substances are required to be the same.
Manufacturers should be aware that these products need to comply with Directive 2001/83/EC Annex I – this sets out the relevant requirements for: Evaluation of absorption, distribution, metabolism, excretion, local tolerance, toxicity, interaction with other devices, medicinal products or other substances and the potential for adverse reactions. Important to note that these must be considered as part of any claim of equivalence.
Medical devices & ancillary medicinal substance: When demonstrating equivalence and reviewing the biological characteristics requirement around the same materials or substances, this applies to any medicinal substance and any excipients or coatings as well.
As these products will be Class III – note the additional requirements on claiming equivalence set out above.
If the product under evaluation contains an ancillary medicinal substance, equivalence cannot be claimed against a device without an ancillary medicinal substance.
Products without an intended medical purpose: equivalence can be claimed against an analogous medical device – if there is clear justification. Otherwise these products are expected to have clinical investigations performed to generate the data. The three characteristics of equivalence should be followed. ‘Clinical benefit’ is interpreted as the need to demonstrate the performance.
Don’t forget to check any relevant common specifications for any additional requirements for that product group!
Documentation:
As set out throughout this blog – documentation is critical. MDCG 2020-5 provides manufacturers with table to utilise for the demonstration of equivalence, we’d encourage you to look and familiarise yourself with the table.
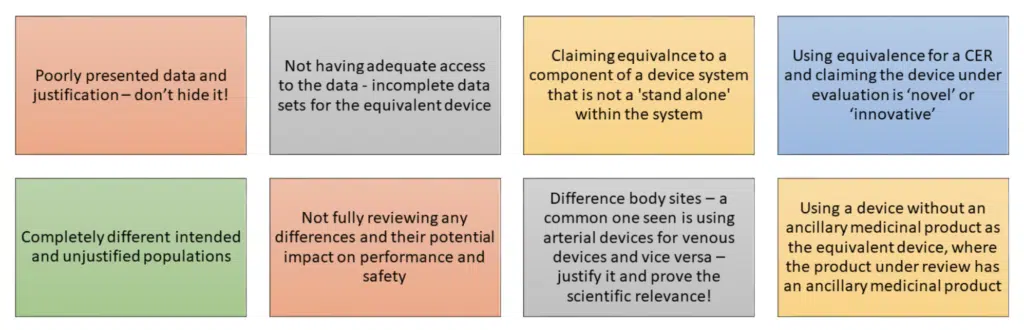
TAKE HOME MESSAGE:
We hope this has helped set out the requirements and considerations when looking to use equivalence in your CER. For the detailed text, refer to the MDR & MDCG 2020-5 and do get in touch if you have any further questions.
For reference: FDA device classes**
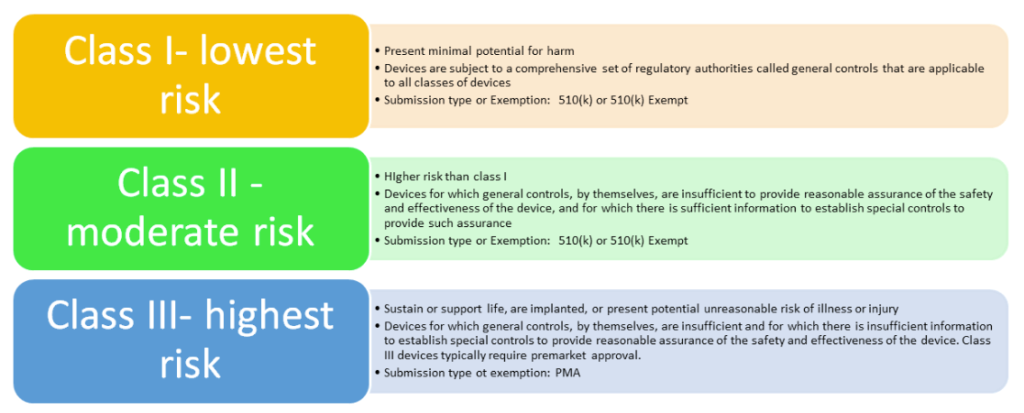
If you wish to place a device on the US market and a Premarket Approval Application is not required – you must submit a 510(k) to the FDA *note there are exemptions & limitations to this ‘.9 of the device classification regulation chapters (21 CFR 862.9, 21 CFR 864.9)’
The main principle of this submission is to demonstrate that your product is as ‘safe and effective’ (substantially equivalent) to a device that is legally marketed in the US. The legally marketed device or devices are referred to as the predicate devices(s).
You cannot market your device until the FDA have deemed it to be substantially equivalent – this decision is usually made within 90 days and is solely based on the information you provide in your submission.
510(k) submission cleared by the FDA are published – usually in the first week of the following month.
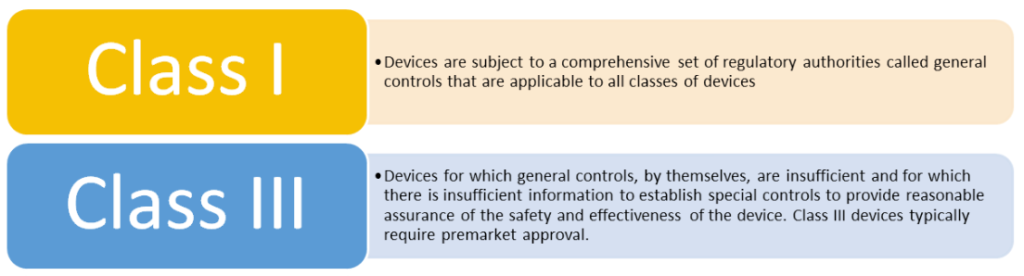
What counts as a predicate device?
It is possible to use more than one predicate,; times this is utilized include:
If there is no appropriate predicate device, then manufacturers will need to submit a De Novo request or for high risk products a Premarket Approval may be required.
The approach to demonstrating equivalence is different to the EU/UK and has two key considerations: intended use and technological characteristics.
Below shows the two different ways in demonstrating this.
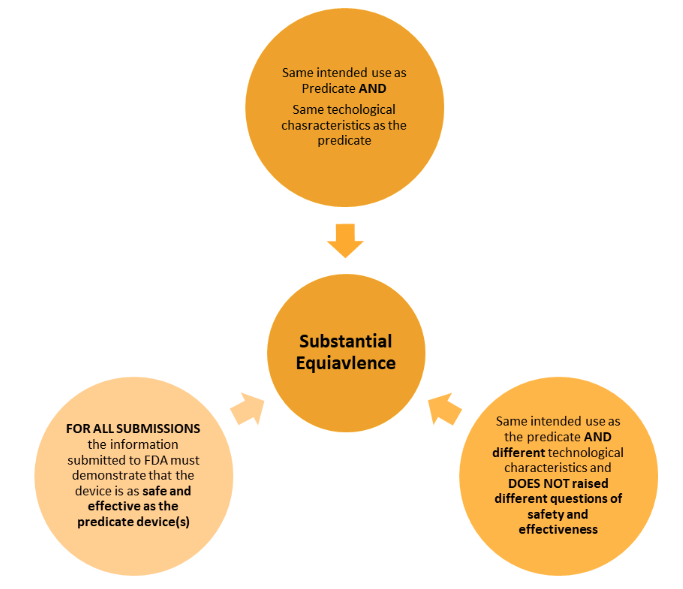
There are less strict requirements within the regulations when compared to the EU approach – however the principles are very much the same. The similarities and the differences must be clearly set out and evaluated.
There must be clear evidence to demonstrate the use of the predicate device(s) is valid and that there is assurance that the safety and effectiveness are substantially equivalent.
The onus is on the manufacturer to ensure this is presented in a robust and scientifically valid way.
These fall in to 2 categories – (1) FDA decision that the device is Class III and cannot be reviewed through 510(k); (2) inadequacies in evidence provided in the submission
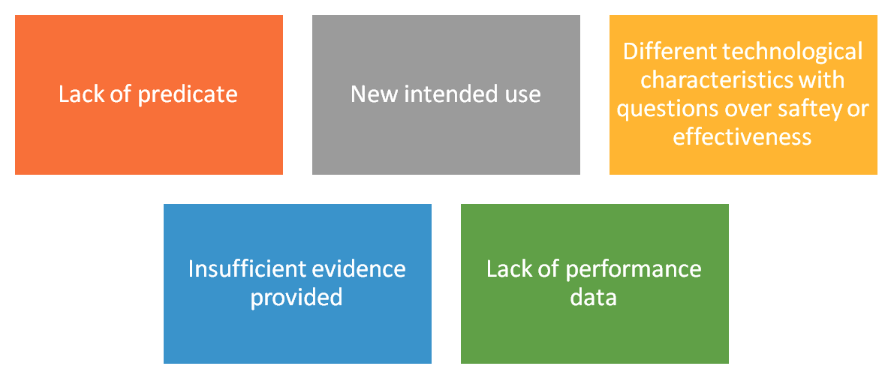
If an NSE decision is given – the manufacturer is given the chance to respond to the FDA determination & the deficiencies noted.
Take home message:
We hope this has helped set out the requirements and considerations when looking to use the FDA’s 510(k) route. For further guidance on a evaluating substantial equivalence for your device you can refer to the FDA’s guidance
Eclevar MedTech a global CRO can help manufacturers understand the requirements for market entry to US. Our team works as an extension team of medical affairs and regulatory affairs sponsor offering customized service with dedicated staffing clinical or regulatory expert with over 20 years’ experience who can help navigate you to the effective solution. Please contact us at clientcare@eclevar.com to learn more.
Subscribe to our newsletter

VISIT US
ECLEVAR FRANCE:
231 rue Saint-Honoré, 75001 Paris, France
ECLEVAR GMBH
ERFURT, Erfurt Hauptbahnhof
4th, 5th floor
Bahnhofstr. 38 Erfurt 99084
ECLEVAR Australia
Umina Beach NSW 2257, Australia
ECLEVAR UK Limited
3rd Floor 207 Regent Street, London, W1B 3HH
CONTACT US