MDCG Guidance documents summary series:
MDCG 2020-10/1 – Safety reporting in clinical investigation of medical devices under the Regulation (EU) 2017/745 and it’s Appendix MDCG 2020-10/2 Clinical Investigation Summary Safety Reporting Form
Introduction
EUDAMED (electronic system referred to in Article 73 of the MDR) was expected to be fully functional in June 2022, instead of 26 May 2021, but this module is still delayed. Per MDR 2017/745 Sponsors of clinical investigation are required to report SAEs to participating Member State(s) via EUDAMED.
MDCG 2020-10 was published to support Sponsors and Competent Authorities in the SAE reporting processes for clinical investigations in the absence of the European EUDAMED.
It “defines Serious Adverse Event (SAE) reporting modalities and includes a summary tabulation reporting format.”
MDCG 2020-10 content
This Guidance covers the changes for SAE reporting requirements under MDR and also highlights PMCF SAE and Vigilance reporting for studies falling under Art. 74.1, which required SAE reporting per national requirements under MDD and AIMDD.
Under AIMDD and MDD, any AE and SAE as outlined in the CIP, whether related to the investigational device, comparator devices, or to study procedures needed to be reported to the CAs of all participating member States – AEs within the final study report and SAEs as per the national requirements of the MS.
Art. 62 and 74.2 studies
- Under MDR 2017/745 any AE and SAE as outlined in the CIP need to be recorded and reported in the final study report, but only SAEs meeting Art. 80.2 are reportable to NCAs, those are:SAE where a relationship to the investigational device, comparator or investigation procedure cannot at least not be excluded,
- Device Deficiencies that might have led to an SAE or
- updates to a) and b)
Attention should be paid, that now under MDR 2017/745 there is a strict differentiation between clinical investigation Art. 80.2 b) reporting via EUDAMED (Art. 73) for a “device deficiency that might have led to a serious adverse event if appropriate action had not been taken, intervention had not occurred, or circumstances had been less fortunate;” and vigilance reporting via EUDAMED Art. 92. of serious incidents. This is valid also for comparator devices.
Clause 79 of the MDR 2017/745 states that “The reporting of serious adverse events or device deficiencies during clinical investigations and the reporting of serious incidents occurring after a device has been placed on the market should be clearly distinguished to avoid double reporting.”
However, reportable events for CE marked medical devices need to be managed by the post-market surveillance/vigilance system (Manufacturer).
PMCF studies
When it comes to PMCF clinical investigation as per Art. 74.1 MDR, awareness of the split requirements is pointed out.
Since these are CE marked device, Vigilance reporting via electronic system (EUDAMED) Art. 92 as per Art. 87 to 90 MDR 2017/745 is required during this investigation (Art. 80.5 MDR), but reporting of serious adverse events where a causal relationship to the preceding investigational procedure has been established (Art. 80.6 MDR) shall be performed as SAE reporting via electronic system (EUDAMED) Art. 73. As above, double reporting shall be avoided.
On page 7 of the Guidance, a description for “preceding investigational procedure” is provided.
Art. 82 studies
MDR Art. 82 “other studies” fall under national requirements, so they are out of scope of the MDR 2017/745, but the Member States can choose to follow the MDCG guidance. Examples of these studies could be:
- CE-marked, observational, not additional burdensome or invasive – Art. 82
- Non-CE marked, novel device, not for indications in Art. 62.1 – Art. 82
- CE-marked, not for indications in Art. 62.1 – Art. 82
MDCG encourages sponsors to check with the applicable Member State which reporting procedures should be applied.
Transition
From 26 May 2021 all the Safety Reporting shall be in accordance with the MDR requirements for new and all ongoing studies, including Art. 74.1 and where applicable Art. 80.2 studies, and the MDCG 2020-10/2 SAE reporting form shall be used.
Medical devices used in clinical trials with medicinal products where the device is non-CE marked, or CE-marked but used outside of the intended use, SAE reporting shall be performed as per the Clinical Trials Regulation (EU 536/2014) AND as per MDR Chapter VI and MDCG 2020-10/1 and 2020-10/2.
If in this kind of studies the Medical device is not assessed for safety and performance this guidance and MDR Art. 80 do not apply. If the device used is CE-marked, MDR Vigilance reporting would apply. Sponsors of these studies should inform the Manufacturer about any incidents. The manufacturer is responsible for the reporting as per Art. 87 to 90 MDR.
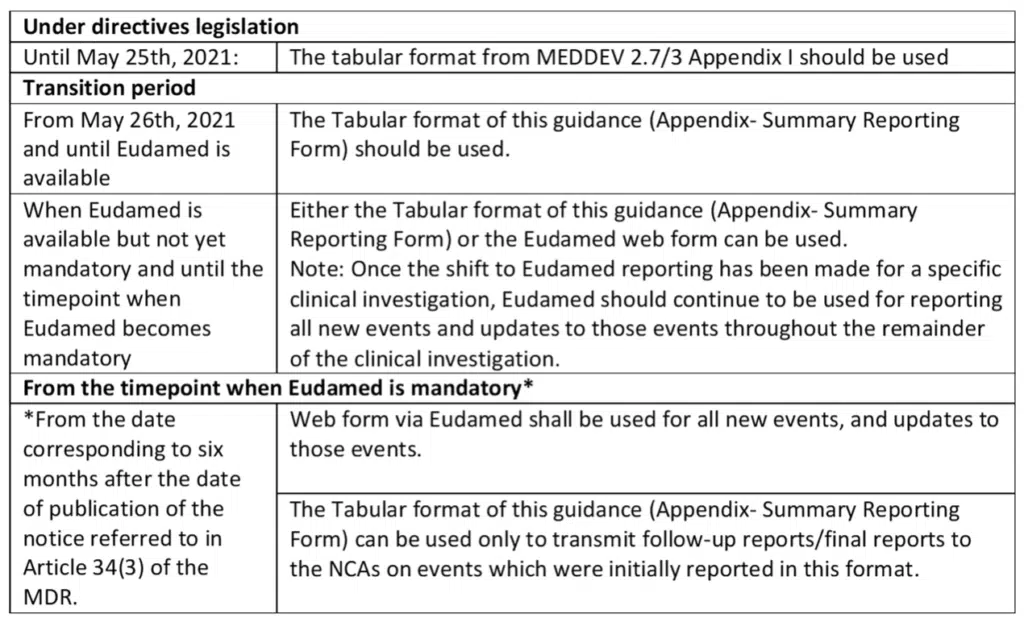
Overview Of Formats To Be Used (from MDCG 2020-10/1 4.2)
Third Countries
Section 5.2 focuses on reportable events occurring in third countries, if the clinical investigation is performed there under the same protocol. This guidance shall apply for third country SAE reporting also.
Additionally, the MDCG provides rules for reporting in Third Countries in ongoing clinical investigation:
- “The NCA shall start receiving the reportable events occurring in Third Countries as soon as the clinical investigation is authorised to start in that Member State.
- Events occurring in Third Countries after the participating European sites have closed shall continue to be reported.”
“Sponsor” and Reporting Timelines
Per Section 6 the Sponsor of the clinical investigation is responsible for Event reporting: “which could be the manufacturer, the legal representative or another person or entity.”
In Section 7 it is stated that reportable events must be reported to all study participating NCAs at the same time.
The start of a clinical investigation in a Member State is described as:
- “For investigations under the directives: When the sponsor is authorized to start the investigation in accordance with the notification procedures in that Member State.
- For investigations started under the MDR: When the sponsor is authorized to start the investigation in that Member State in accordance with the provisions laid out in the MDR.”
Sponsor Reporting
Article | Event | Report To | Timeline (MDCG 2020-10-1) | |
80 (2) a | SAEs (Art 2 number 58) where a relationship with the device, comparator, or study procedure cannot be excluded
| EEA Member State CAs / other countries MDCG 2020-10/2 | MDCG 2020-10/1: imminent risk of death, serious injury, or serious illness and that requires prompt remedial action for other patients/subjects, users or other persons or a new finding to it: Immediately, but not later than 2 calendar days after awareness by sponsor Any other reportable events or a new finding/update to it: Immediately, but not later than 7 calendar days | |
80 (2) b | Device Deficiency with potential to SA(D)E
| EEA Member State CAs / other countries MDCG 2020-10/2 | MDCG 2020-10/1: imminent risk of death, serious injury, or serious illness and that requires prompt remedial action for other patients/subjects, users or other persons or a new finding to it: Immediately, but not later than 2 calendar days after awareness by sponsor Any other reportable events or a new finding/update to it: Immediately, but not later than 7 calendar days | |
80 (2) c | any new findings in relation to any event referred to in points (a) and (b) | EEA Member State CAs / other countries MDCG 2020-10/2 | MDCG 2020-10/1: imminent risk of death, serious injury, or serious illness and that requires prompt remedial action for other patients/subjects, users or other persons or a new finding to it: Immediately, but not later than 2 calendar days after awareness by sponsor Any other reportable events or a new finding/update to it: Immediately, but not later than 7 calendar days | |
MDCG 2020-10/1, 10.2.18 Unanticipated SADE | EEA Member State CAs / other countries MDCG 2020-10/2 / via Electronic System (Art. 73) | |||
Investigator reporting
Article | Event as outlined in CIP | Report To | Timeline (MDCG 2020-10-1) |
80 (1) a | Adverse Event (AE) | Sponsor | immediately, but not later than 3 calendar days/ as outlined in the Protocol |
80 (1) b | Serious Adverse Event (SAE) | Sponsor | immediately, but not later than 3 calendar days/ as outlined in the Protocol |
80 (1) c | Device Deficiency with potential to SA(D)E | Sponsor | immediately, but not later than 3 calendar days/ as outlined in the Protocol |
80 (1) d | new findings related to Events in a to c | Sponsor | immediately, but not later than 3 calendar days/ as outlined in the Protocol |
Causality Assessment
The Causality assessment needs to be performed by the Investigator in first place, but also by the Sponsor as the overall responsible person for the clinical investigation.
MDCG 2020-10/1 provides a clear guidance with practical explanations for the causality (investigational device, comparator, procedure):
- Not related – relationship can be excluded
- Possible – relationship cannot be excluded
- Probable – relationship seems “relevant and/or the event cannot be reasonably explained by another cause”
- Causal relationship – relationship is beyond reasonable doubt
“Where an investigator assessment is not available and/or the sponsor remains uncertain about classifying the serious adverse event, the sponsor should not exclude the relatedness; the event should be classified as “possible” and the reporting not be delayed.
Particular attention shall be given to the causality evaluation of unanticipated serious adverse events. The occurrence of unanticipated events related could suggest that the clinical investigation places subjects at increased risk of harm than was to be expected beforehand.”
Clinical Investigation Summary Safety Reporting Form MDCG 2020-10/2 – Reporting Form and Completion Guidelines
Regarding the SAE reporting form, MDCG states: “English is the recommended language for the reporting form. The report form can be modified in any applicable software (not only Microsoft Excel) but the file needs to be compatible with Microsoft Excel when sent to the participating NCAs”.
It shall be completed for each new event or new information on an ongoing event and submitted to all participating NCAs at the same time. The table is a cumulative overview of all reportable events in a clinical investigation, and nothing should be deleted during the investigation.
In section 10.1 examples and explanations for completing the form header is given, and in section 10.2 precise explanation on the completion of the event details in the forms.
Other helpful MDCG Guidance
- MDCG 2021-6 Regulation (EU) 2017/745 – Questions & Answers regarding clinical investigation
- MDCG 2021-8 Clinical investigation application/notification documents, see also our blog: Summary of the MDCG Guidance Document MDCG 2021-8.
- MDCG 2021-20 Instructions for generating CIV-ID for MDR Clinical Investigations, see also our blog: Summary of the MDCG Guidance Document MDCG 2021-20
- MDCG 2021-28 Substantial modification of clinical investigation under Medical Device Regulation
- The MDCG 2021-1 Rev.1 Guidance on harmonised administrative practices and alternative technical solutions until EUDAMED is fully functional (respectively referrals to: Art. 70, Art. 73, Art. 74, Art. 75, Art. 76, Art. 77, Art. 78, Art. 80), see also our blog: Summary of the MDCG Guidance Document MDCG 2021-20
- MDCG 2019-9 Rev.1 Summary of safety and clinical performance A guide for manufacturers and notified bodies
Helpful guidance is provided for the Classification of Devices and Combination Products Medical Device and Medicinal Product:
- MDCG 2022-5 Guidance on the borderline between medical devices and medicinal products under Regulation (EU) 2017/745 on medical devices
- MDCG 2021-24 Guidance on classification of medical devices
- Exchange of information between medical device competent authorities on borderline and classification cases Helsinki Procedure 2021
In addition, helpful guidance is provided for “actors” registration under MDR:
- MDCG 2020-15: MDCG Position Paper on the use of the EUDAMED actor registration module and of the Single Registration Number (SRN) in the Member States
- MDCG 2021-13 rev.1: Questions and answers on obligations and related rules for the registration in EUDAMED of actors other than manufacturers, authorised representatives, and importers subject to the obligations of Article 31 MDR and Article 28 IVDR
Thank you for your time. If you found this interesting, please check-out for out next blog on the MDCG Document summaries: MDCG 2029-9 Rev.1 – Summary of safety and clinical performance A guide for manufacturers and notified bodies

