Women’s reproductive organs maintain their anatomic position in the pelvis through an intricate network of support structures, including connective tissues, ligaments, and muscles.
Pelvic organ prolapse (POP) results when 1 or more of these support structures are compromised due to childbirth, aging, and/or increased intra-abdominal pressure. The following 1 demonstrates different risk factors which result in POP.
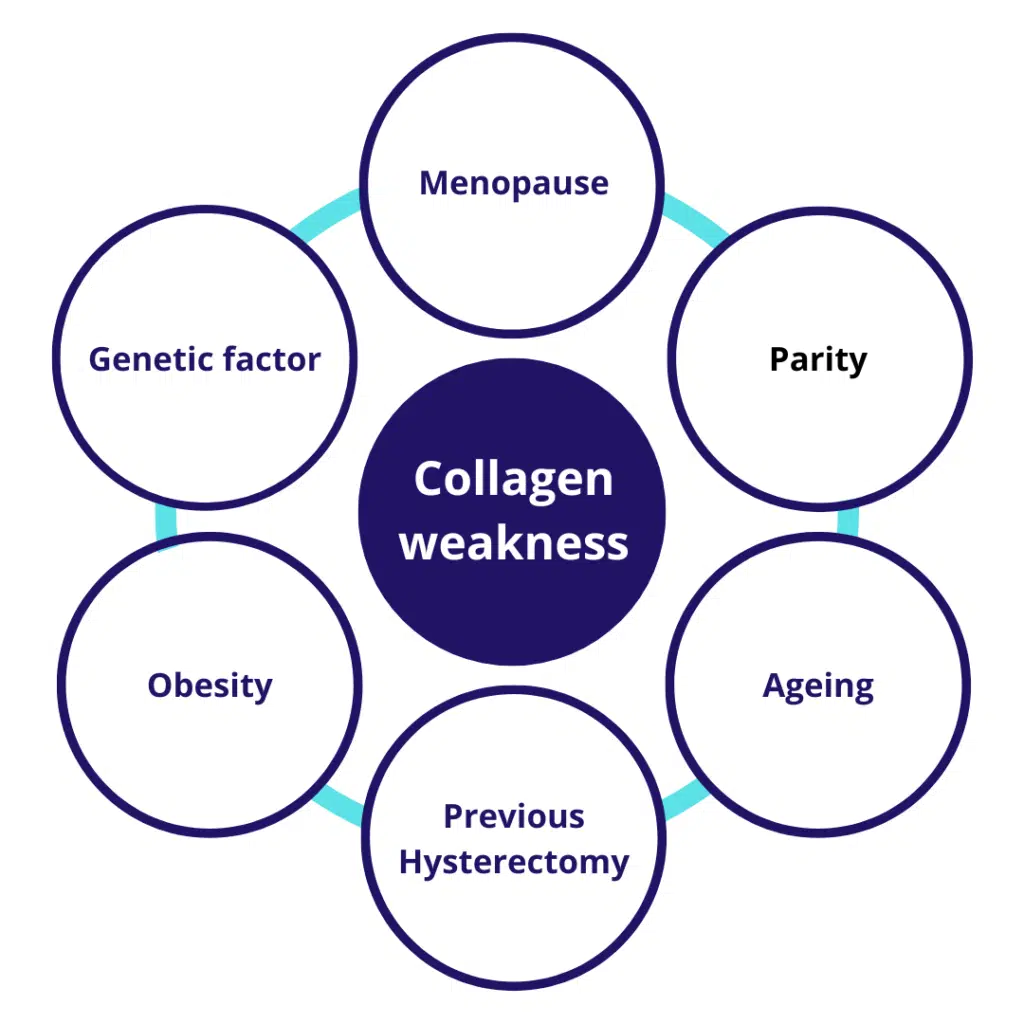

As a result, the vagina descends, and normal pelvic function may be lost to a variable extent. Routine gynaecological examination can detect a vaginal bulge in approximately 50% of patients. However, POP is only symptomatic in 3% to 6% of women, with the most common symptom being a sensation of vaginal bulging. POP is one of several pelvic floor disorders. These often coexist, and their symptoms overlap.
As a consequence, clinical assessment for POP also includes screening for urinary, vaginal, or bowel dysfunction, including sexual dysfunction and pain. Although POP treatments can occasionally correct other pelvic symptoms, they may also exacerbate them. For example, stress urinary incontinence can be unmasked after surgery for POP. Other pelvic floor disorders and possible effects on them warrant discussion with patients seeking care for POP.
Types of prolapse include:
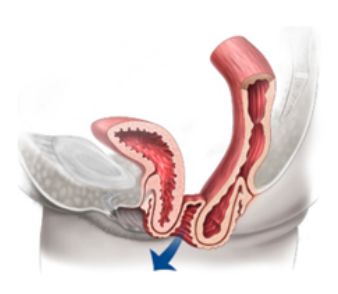
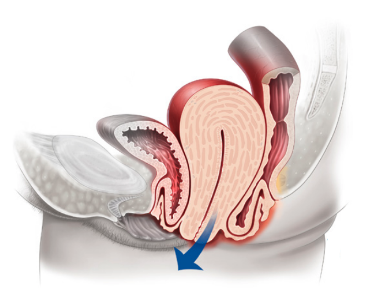

Anterior vaginal wall prolapse (i.e., cystocoele (bladder descends), see Figure 3, urethrocoele
(urethra descends), paravaginal defect (pelvic fascia defect)
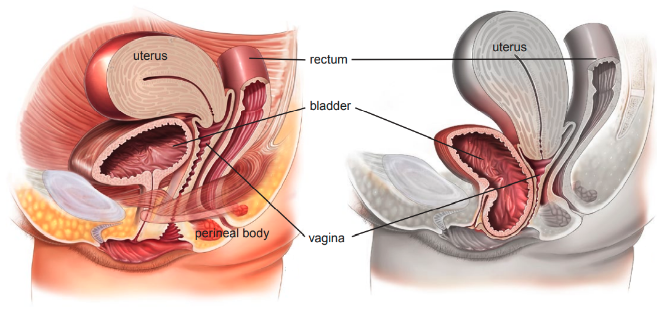

Posterior vaginal wall prolapse (i.e., enterocoele (smallbowel descends), rectocoele (rectum descends), see Figure 4, perineal deficiency).


Since the introduction of polypropylene mesh in the middle of the 20th century, many mesh materials and configurations for specific surgical procedures have been developed.
Surgical mesh may be used for repair of diaphragmatic hernias, urinary incontinence in women (female slings), genitourinary prolapse (vaginal mesh and sacrocolpopexy), rectal prolapse (rectopexy), and postprostatectomy male urinary incontinence (male slings).
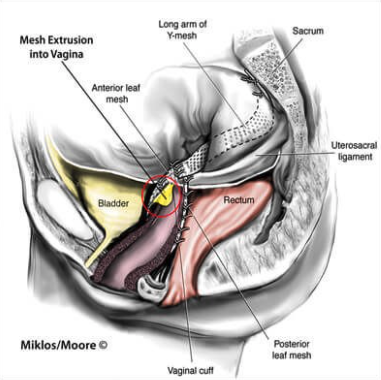

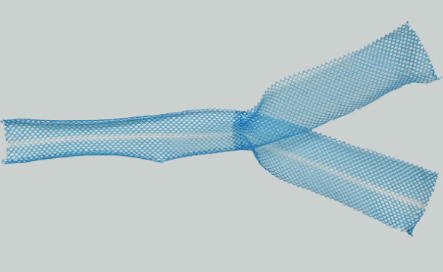
Figure 5 Photograph shows sacrocolpopexy polypropylene mesh with the Y configuration.
Women with prolapse commonly have a variety of pelvic floor symptoms. Symptoms of prolapse include pelvic heaviness; a bulge, lump, or protrusion coming down from the vagina; a dragging sensation in the vagina; and backache. Symptoms of bladder, bowel, or sexual dysfunction are frequently present. For example, women may need to reduce the prolapse by using their fingers to push the prolapse up to facilitate urinary voiding or defecation.
These symptoms may be directly related to the prolapsed organ, for example, poor urinary stream when a cystocoele is present, or obstructed defecation when a rectocele is present. They may also be independent of the prolapse, for example, symptoms of overactive bladder when a cystocoele is present.
Examples of measures for success based on case studies:
Anatomy (correction of prolapse on physical examination)
The need for eventual reoperation or pessary device (recurrence of prolapse).
The objective success is based on the anatomy, which uses the pelvic organ prolapse-quantification (POP-Q) system1.
The medical device: The medical devices is a surgical mesh intended for: Pelvic Organ Prolapse: Cystocele, hysterocele, and/or Rectocele
Problem: As shown by the Clinical Evaluation Report, intended claims on clinical safety and performance are not sufficiently supported by existing clinical evidence. To maintain a “implant “in the European market and obtain the renewal of the reimbursement statue in France, the sponsor has to conduct a PMCF study to generate sufficient clinical data in accordance with “Chapter VI – Clinical Evaluation and Clinical Investigations, specifically sections 62 – 82”. As well as ISO 14155:2020.
Objectives
As with many surgeries performed with the goal of alleviating a patient’s symptoms and improving quality of life, there exists a dichotomy in the definition of success after surgery for Pelvic Organ Prolapse. ECLEVAR MEDTECH collected in France Real world evidence study to gather long-term clinical follow-up data. ECLEVAR was responsible on drafting the clinical evaluation process for the client.
Objective measures for success was based upon:
Anatomy (correction of prolapse on physical examination)
The need for eventual reoperation or pessary device (recurrence of prolapse).
Subjective assessment include:
Patient self- reported degree of bother, presence or absence of a bulge, level of activity, dyspareunia, quality of life and/or overall satisfaction

Subscribe to our newsletter

VISIT US
ECLEVAR FRANCE:
231 rue Saint-Honoré, 75001 Paris, France
ECLEVAR GMBH
ERFURT, Erfurt Hauptbahnhof
4th, 5th floor
Bahnhofstr. 38 Erfurt 99084
ECLEVAR Australia
Umina Beach NSW 2257, Australia
ECLEVAR UK Limited
3rd Floor 207 Regent Street, London, W1B 3HH
CONTACT US